SUMMARY: Radium Ra 223 dichloride (XOFIGO®) is a bone seeking alpha emitter that selectively targets areas of increased bone turnover. It induces double–stranded DNA breaks and has a very limited range path and quickly loses energy within a short distance of its source. This results in less damage to the adjacent healthy tissue. Further, unlike the dreaded Ra 226 which was first isolated by Madame Curie, XOFIGO® has a short half life of 11.4 days and rapidly decays preventing radiation exposure. In a randomized, double-blind phase III trial, 921 patients with Castrate Resistant Prostate Cancer (CRPC) who had progressed on or had not received TAXOTERE® (Docetaxel) for a variety of reasons, were randomly assigned in a 2:1 ratio to receive either XOFIGO®, with best supportive care or PLACEBO with best supportive care. Patients with visceral metastases were excluded. The primary endpoint was overall survival and secondary endpoints included time to first symptomatic skeletal event, time to increase in total alkaline phosphatase level and PSA level. There was a significant increase in the median overall survival in the XOFIGO® group compared to placebo group with a 30% reduction in the risk of death (14.9 months vs 11.3 months, HR=0.70, P<0.001). All secondary endpoints favored XOFIGO® as well. All adverse events were lower in the XOFIGO® group and myelosuppression was minimal. Unlike the bone seeking beta emitters, Strontium-89 and Samarium-153, XOFIGO®, an alpha emitter, is the only agent that has been shown to improve overall survival. Studies are underway evaluating the efficacy of chemotherapy in combination with XOFIGO®, in patients with CRPC with bone metastases. Parker C, Nilsson S, Heinrich D, et al. N Eng J Med 2013;369:213-23
Month: July 2013
LUX-Lung 3 A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations
SUMMARY: GILOTRIF® (Afatinib) is an oral, irreversible blocker of the ErbB family which includes EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4. The approval of GILOTRIF® was based on a multi-center, international, open-label, randomized, phase III trial, in which 345 patients with Stage IIIB (wet)/IV lung adenocarcinoma, with tumors demonstrating Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations, as detected by an FDA-approved test, were enrolled in a 2:1 ratio. Patients were randomized to receive GILOTRIF® 40 mg orally once daily (n=230) or ALIMTA® (Pemetrexed)/Cisplatin (n=115). Patients were stratified according to EGFR mutation status (exon 19 deletion vs. exon 21 L858R vs. ‘other’) and race (Asian vs. non-Asian). The primary endpoint was Progression Free Survival (PFS). The median PFS in the GILOTRIF® group was 11.1 months and 6.9 months in the chemotherapy group (HR= 0.58, P<0.001). In patients whose tumors demonstrated EGFR mutations, the median PFS was 13.6 months in the GILOTRIF® arm and 6.9 months in the chemotherapy arm (HR= 0.47, P<0.0001). Objective response rates were 50.4% and 19.1% in the GILOTRIF® and chemotherapy groups respectively. There was no statistically significant difference in overall survival between the two treatment groups. The most frequent adverse reactions in the GILOTRIF® group were skin rash, pruritus, stomatitis, diarrhea and decreased appetite. The authors concluded that GILOTRIF® is better than chemotherapy in the first line treatment of EGFR mutant Non Small Cell Lung Cancer patients. However, it remains to be seen if this agent is superior to TARCEVA® (Erlotinib) and IRESSA® (Gefitinib). Yang JC, Shuler M, Yamamoto N, et al. J Clin Oncol 2012;30(18,Suppl):abstract LBA 7500.
GILOTRIF® for EGFR mutation positive Lung Cancer
GILOTRIF® (Afatinib) is an oral, irreversible blocker of the ErbB family which includes EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4. The FDA approved GILOTRIF® based on a multicenter, randomized phase III trial (LUX-Lung 3) in which GILOTRIF® trumped chemotherapy when administered to chemonaive patients with EGFR mutation positive Non Small Lung Cancer. We have yet another targeted oral agent besting chemotherapy. This is another milestone in the Lung Cancer treatment paradigm.
GILOTRIF® (Afatinib)
The FDA on July 12, 2013 approved the use of GILOTRIF® tablets for the first-line treatment of patients with metastatic Non-Small Cell Lung Cancer (NSCLC) whose tumors have Epidermal Growth Factor Receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test. The FDA also approved THERASCREEN, a test provided by QIAGEN, for the detection of EGFR exon 19 deletions or exon 21 (L858R) substitution mutations. GILOTRIF® is a product of Boehringer Ingelheim Pharmaceuticals, Inc.
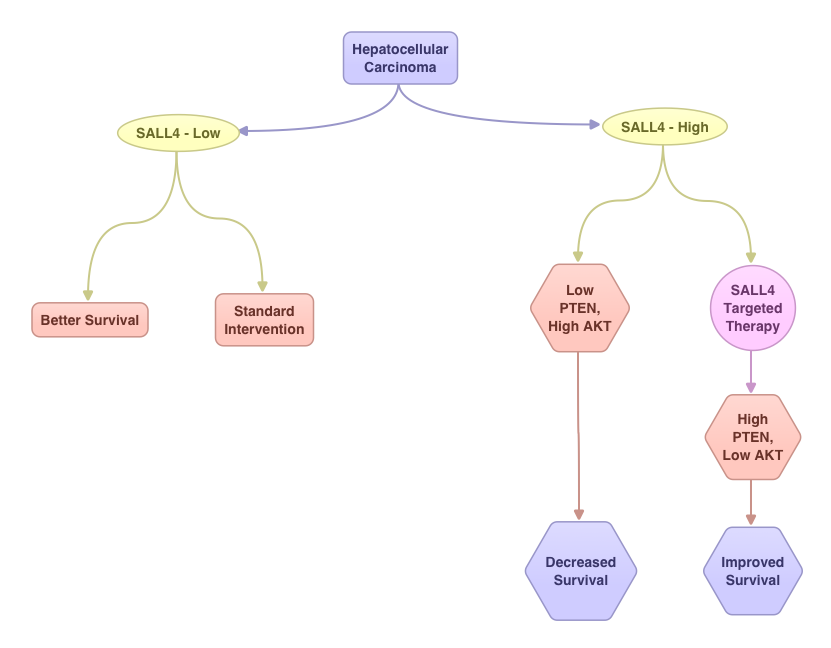
Oncofetal Gene SALL4 in Aggressive Hepatocellular Carcinoma
SUMMARY:Hepatocellular Carcinoma (HCC) originates from hepatocytes and is the sixth most common cancer and third leading cause of cancer related death worldwide. Chronic liver injury and cirrhosis have been implicated as important risk factors. This may result from infections with Hepatitis B and C, heavy alcohol consumption, exposure to Aflatoxin, a potent carcinogen produced by Aspergillus species and non alcoholic fatty liver disease (NAFLD) seen in patients with obesity and diabetes. The underlying liver disease contributing to tumorigenesis adds to the molecular complexity of HCC. The standard intervention for advanced stage HCC has been multi receptor Tyrosine Kinase Inhibitor, NEXAVAR® (Sorafenib). Alpha Feto Protein (AFP) is normally produced by the liver and yolk sac of a fetus during pregnancy and decreases soon after birth. AFP has been used as a serological test for HCC surveillance. In this study, the authors evaluated the role of SALL4, an oncofetal gene, which is expressed in the fetal liver but silenced in the adult liver. In HCC, SALL4 is re-expressed in the tumor tissue and may play an important role in hepatocarcinogenesis and may also portend poor outcomes. SALL4 may therefore serve as an important biomarker and molecular target. Targeting SALL4 could increase the expression of tumor suppressor gene PTEN and block the PI3K survival signaling pathway, by dephosphorylating AKT. The authors concluded that testing hepatic tumor tissue for SALL4 at the time of diagnosis may have prognostic value and may identify patient groups who are likely to benefit from SALL4 targeted therapy. Yong KJ, Gao C, Lim J, et al. N Engl J Med 2013; 368:2266-2276