SUMMARY: Circulating tumor cells (CTCs) are epithelial cells that are shed into the circulation from a primary or metastatic tumor. After being shed, CTCs can remain in the circulation or undergo apoptosis. 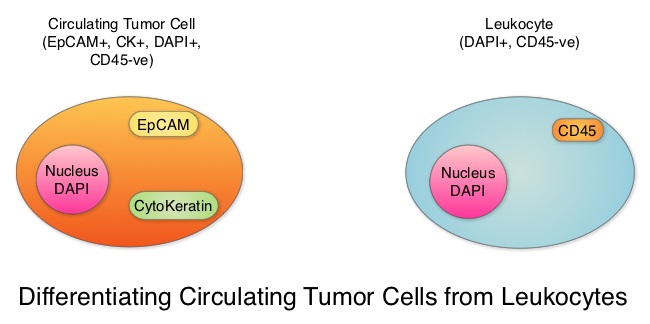 Evaluation of CTCs during the course of disease has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs. One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK positive, CD45 negative and DAPI positive. In essence, CTC assessment is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. The authors in this study conducted a pooled analysis to assess the clinical validity of CTCs in patients with MBC, as previously published studies reported contradictory results and were unable to ascertain whether enumeration of CTCs had better prognostic value than the traditional clinical and pathological features of the tumor and serum tumor markers. Data was gathered on 1944 patients with MBC, who had participated in clinical trials at 17 centers between 2003 and 2012. Participants in these studies were starting a new line of therapy (predominantly chemotherapy), and these studies had CTC quantification using CellSearch® platform, before start of new treatment (baseline), data for Progression Free Survival, Overall Survival or both. Using accepted statistical methodologies, the authors noted the following findings-
Evaluation of CTCs during the course of disease has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs. One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK positive, CD45 negative and DAPI positive. In essence, CTC assessment is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. The authors in this study conducted a pooled analysis to assess the clinical validity of CTCs in patients with MBC, as previously published studies reported contradictory results and were unable to ascertain whether enumeration of CTCs had better prognostic value than the traditional clinical and pathological features of the tumor and serum tumor markers. Data was gathered on 1944 patients with MBC, who had participated in clinical trials at 17 centers between 2003 and 2012. Participants in these studies were starting a new line of therapy (predominantly chemotherapy), and these studies had CTC quantification using CellSearch® platform, before start of new treatment (baseline), data for Progression Free Survival, Overall Survival or both. Using accepted statistical methodologies, the authors noted the following findings-
1) At baseline prior to starting treatment, 47% (N=911) patients had 5 or more CTCs per 7•5 mL of peripheral blood, suggesting more aggressive disease and this was associated with decreased Progression Free Survival (P<0•0001) and Overall Survival (P<0•0001), compared with patients with less than 5 CTCs per 7•5 mL at baseline.
2) At 3-5 weeks after start of treatment (1-2 cycles of treatment), when adjusted for baseline CTC count, 5 or more CTCs per 7•5 mL of peripheral blood was associated with shortened Progression Free Survival (P<0•0001) and Overall Survival (P<0•0001), suggesting that these patients were treatment resistant.
3) An early decrease in the CTC at week 3-5, from a high baseline of 5 or more CTCs per 7•5 mL to less than 5 CTCs per 7.5 ml was associated with significantly longer Progression Free Survival and Overall Survival.
4) Enumeration of CTCs was a better predictor of prognosis than mucin based serum biomarkers such as CEA and CA15-3.
The prognostic value of high CTC count at baseline and at 3-5 weeks of treatment, on Progression Free Survival and Overall Survival was maintained in all subgroups tested, regardless of breast cancer subtypes and type of therapy patients received. Based on this pooled analysis, with the largest assessment of CTC enumeration in MBC patients, the authors concluded that CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course, allowing customized care. Further, CTC enumeration, unlike serum tumor markers, correlates with clinical and pathological characteristics. Bidard F, Peeters DJ, Fehm T, et al. The Lancet Oncology 2014;15:406-414

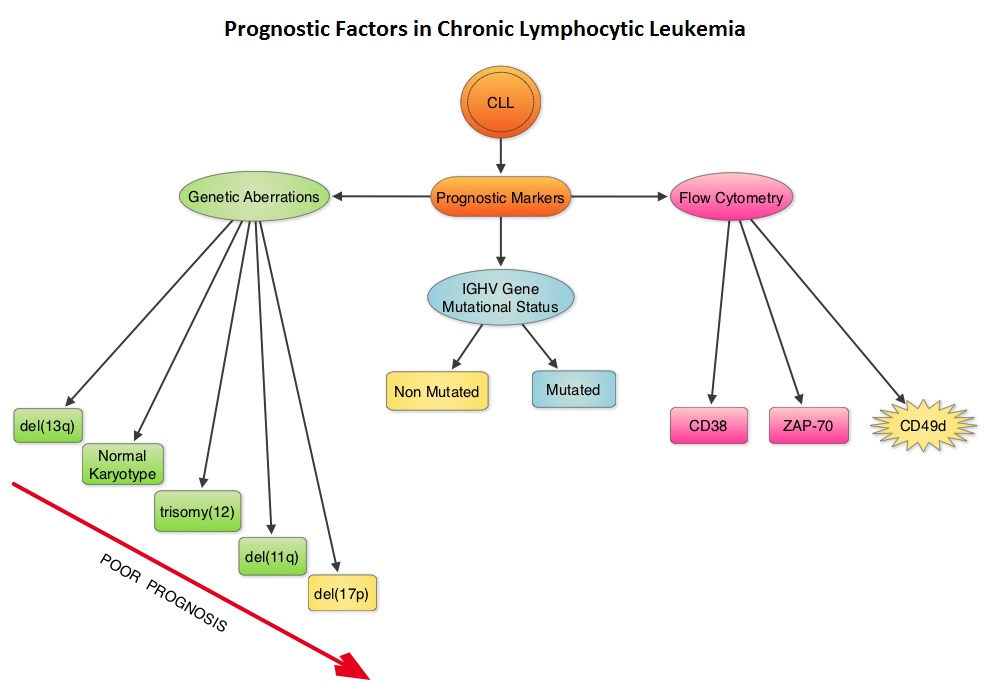 Retrospective studies have suggested that patients with CLL whose leukemic cells have clonotypically rearranged immunoglobulin genes in germline configuration (Unmutated IGHV gene) demonstrated more aggressive disease and shorter survival time compared to those patients with somatic hypermutations in their IGHV genes (Mutated IGHV gene). Expression of two flow cytometry based biomarkers, CD38 (surface marker) and ZAP-70 (intracytoplasmic protein), have been associated with poor outcomes. As we learn more about the pathobiology of CLL, it is becoming clear that survival of CLL cells is dependent not only on their intracellular defects but also on the microenvironment. CD49d, an integrin, is a surface molecule, detected by flow cytometry. CD49d expression promotes microenvironment mediated proliferation of CLL cells and has been associated with shortened survival. The authors conducted this multicenter analysis to evaluate the prognostic utility of CD49d in CLL, in comparison with CD38 and ZAP-70. The authors analysed the data of 2972 patients from 9 clinical trials. All these studies had data on CD49d expression of CLL cells by flow cytometry and reported the association between CD49d expression and Overall survival and/or Treatment Free Survival and/or Progression Free Survival. Patients with 30% or more of CLL cells expressing CD49d were considered CD49d positive. In this pooled analysis, CD49d positive patients had a significantly lower Overall Survival both at 5 years (87% vs 94%) and 10 years (62% vs 84%) compared with CD49d negative patients (P<0.001). Further, CD49d positive patients more likely required treatment, suggesting that these patients had a lower probability of remaining treatment free at both 5 years (42% vs 68%) and 10 years (24% vs 50%), compared with CD49d negative patients. When other variables were taken into consideration, CD49d was the only flow cytometry based marker which independently predicted Overall Survival with greater prognostic relevance than CD38 and ZAP-70. The authors concluded that CD49d expression and IGHV gene mutational status may be the strongest predictors of Overall Survival and Treatment Free Survival in patients with CLL and should be a part of routine baseline testing at the time of diagnosis. Bulian P, Shanafelt TD, Fegan C, et al. J Clin Oncol 2014;32:897-904
Retrospective studies have suggested that patients with CLL whose leukemic cells have clonotypically rearranged immunoglobulin genes in germline configuration (Unmutated IGHV gene) demonstrated more aggressive disease and shorter survival time compared to those patients with somatic hypermutations in their IGHV genes (Mutated IGHV gene). Expression of two flow cytometry based biomarkers, CD38 (surface marker) and ZAP-70 (intracytoplasmic protein), have been associated with poor outcomes. As we learn more about the pathobiology of CLL, it is becoming clear that survival of CLL cells is dependent not only on their intracellular defects but also on the microenvironment. CD49d, an integrin, is a surface molecule, detected by flow cytometry. CD49d expression promotes microenvironment mediated proliferation of CLL cells and has been associated with shortened survival. The authors conducted this multicenter analysis to evaluate the prognostic utility of CD49d in CLL, in comparison with CD38 and ZAP-70. The authors analysed the data of 2972 patients from 9 clinical trials. All these studies had data on CD49d expression of CLL cells by flow cytometry and reported the association between CD49d expression and Overall survival and/or Treatment Free Survival and/or Progression Free Survival. Patients with 30% or more of CLL cells expressing CD49d were considered CD49d positive. In this pooled analysis, CD49d positive patients had a significantly lower Overall Survival both at 5 years (87% vs 94%) and 10 years (62% vs 84%) compared with CD49d negative patients (P<0.001). Further, CD49d positive patients more likely required treatment, suggesting that these patients had a lower probability of remaining treatment free at both 5 years (42% vs 68%) and 10 years (24% vs 50%), compared with CD49d negative patients. When other variables were taken into consideration, CD49d was the only flow cytometry based marker which independently predicted Overall Survival with greater prognostic relevance than CD38 and ZAP-70. The authors concluded that CD49d expression and IGHV gene mutational status may be the strongest predictors of Overall Survival and Treatment Free Survival in patients with CLL and should be a part of routine baseline testing at the time of diagnosis. Bulian P, Shanafelt TD, Fegan C, et al. J Clin Oncol 2014;32:897-904 The CDC updated their recommendations in 2008 and recommended HBV screening for patients receiving cytotoxic chemotherapy or immunotherapy. The American Society of Clinical Oncology in 2010 rendered a Provisional Clinical Opinion (PCO) suggesting that there was insufficient evidence to recommend routine screening for HBV in cancer patients,but screening may be considered for patient populations at high risk or for those who are to receive highly immunosuppressive therapies including anti-CD20 monoclonal antibody therapy such as Rituximab (RITUXAN®). To evaluate compliance with these recommendations, the authors in this study retrospectively reviewed charts of patients with Low grade Non Hodgkins Lymphoma at a teritiary care center and documented the various studies performed, as a part of the pretreatment workup, between January 2005 and December 2011. They noted that only 19% of the total patients and 25% of the patients who received RITUXAN® had HBV screening done. The authors concluded that this was a significant deviation from the recommended guidelines and these findings resulted in the implementation of stricter measures for HBV screening at this teritiary care center. Screening for HBV should include testing for Hepatitis B surface antigen (HBsAg), Antibody to Hepatitis B core antigen (anti-HBc) and Antibody to Hepatitis B surface antigen (Anti-HBs). Patients positive for HBsAg and anti-HBc as well as those who are negative for HBsAg and positive for anti-HBc, should have testing for HBV viral load using serum HBV DNA and those without active disease should receive prophylactic antiviral therapy and be closely monitored for HBV reactivation. Prophylaxis is usually started one week before initiating chemotherapy and continued for at least 6 months after completion of chemotherapy, although the actual duration of prophylactic antiviral therapy remains unclear. If HBV reactivation is noted, chemotherapy should be immediately discontinued. Given the prevalence of chronic Hepatitis B in the United States, screening for HBV should become a routine part of pretreatment evaluation in cancer patients. Abbi KK, Gorris M, Skeel RT. Am J Ther. 2013;June 28.
The CDC updated their recommendations in 2008 and recommended HBV screening for patients receiving cytotoxic chemotherapy or immunotherapy. The American Society of Clinical Oncology in 2010 rendered a Provisional Clinical Opinion (PCO) suggesting that there was insufficient evidence to recommend routine screening for HBV in cancer patients,but screening may be considered for patient populations at high risk or for those who are to receive highly immunosuppressive therapies including anti-CD20 monoclonal antibody therapy such as Rituximab (RITUXAN®). To evaluate compliance with these recommendations, the authors in this study retrospectively reviewed charts of patients with Low grade Non Hodgkins Lymphoma at a teritiary care center and documented the various studies performed, as a part of the pretreatment workup, between January 2005 and December 2011. They noted that only 19% of the total patients and 25% of the patients who received RITUXAN® had HBV screening done. The authors concluded that this was a significant deviation from the recommended guidelines and these findings resulted in the implementation of stricter measures for HBV screening at this teritiary care center. Screening for HBV should include testing for Hepatitis B surface antigen (HBsAg), Antibody to Hepatitis B core antigen (anti-HBc) and Antibody to Hepatitis B surface antigen (Anti-HBs). Patients positive for HBsAg and anti-HBc as well as those who are negative for HBsAg and positive for anti-HBc, should have testing for HBV viral load using serum HBV DNA and those without active disease should receive prophylactic antiviral therapy and be closely monitored for HBV reactivation. Prophylaxis is usually started one week before initiating chemotherapy and continued for at least 6 months after completion of chemotherapy, although the actual duration of prophylactic antiviral therapy remains unclear. If HBV reactivation is noted, chemotherapy should be immediately discontinued. Given the prevalence of chronic Hepatitis B in the United States, screening for HBV should become a routine part of pretreatment evaluation in cancer patients. Abbi KK, Gorris M, Skeel RT. Am J Ther. 2013;June 28. Radium Ra 223 dichloride (XOFIGO®) is a bone seeking alpha particle emitter and by virtue of its chemical similarity to calcium is preferentially taken up by the bone and forms complexes with bone mineral, hydroxyapatite, in areas where there is increased bone turnover, such as bone metastases. XOFIGO® induces double stranded DNA breaks resulting in antitumor effects and has a very short range in tissues (around 2 and 10 cells), quickly losing energy, compared to beta or gamma radiation. The end result is less damage to the adjacent healthy tissues. Further, unlike Ra-226 which was first isolated by Madame Curie, XOFIGO® has a short half life of 11.4 days and rapidly decays, preventing significant radiation exposure. The ALSYMPCA (ALpharadin in SYMptomatic Prostate CAncer patients) study is a randomized, double-blind, phase III trial, in which 921 patients with Castrate Resistant Prostate Cancer (CRPC) with 2 or more symptomatic bone metastases and no known visceral metastases, were randomly assigned in a 2:1 ratio to receive either XOFIGO® along with best supportive care (N=614) or placebo with best supportive care (N=307). Enrolled patients either progressed on or had not received TAXOTERE® (Docetaxel) for a variety of reasons and 42% of the enrolled patients were chemotherapy naïve.
Radium Ra 223 dichloride (XOFIGO®) is a bone seeking alpha particle emitter and by virtue of its chemical similarity to calcium is preferentially taken up by the bone and forms complexes with bone mineral, hydroxyapatite, in areas where there is increased bone turnover, such as bone metastases. XOFIGO® induces double stranded DNA breaks resulting in antitumor effects and has a very short range in tissues (around 2 and 10 cells), quickly losing energy, compared to beta or gamma radiation. The end result is less damage to the adjacent healthy tissues. Further, unlike Ra-226 which was first isolated by Madame Curie, XOFIGO® has a short half life of 11.4 days and rapidly decays, preventing significant radiation exposure. The ALSYMPCA (ALpharadin in SYMptomatic Prostate CAncer patients) study is a randomized, double-blind, phase III trial, in which 921 patients with Castrate Resistant Prostate Cancer (CRPC) with 2 or more symptomatic bone metastases and no known visceral metastases, were randomly assigned in a 2:1 ratio to receive either XOFIGO® along with best supportive care (N=614) or placebo with best supportive care (N=307). Enrolled patients either progressed on or had not received TAXOTERE® (Docetaxel) for a variety of reasons and 42% of the enrolled patients were chemotherapy naïve.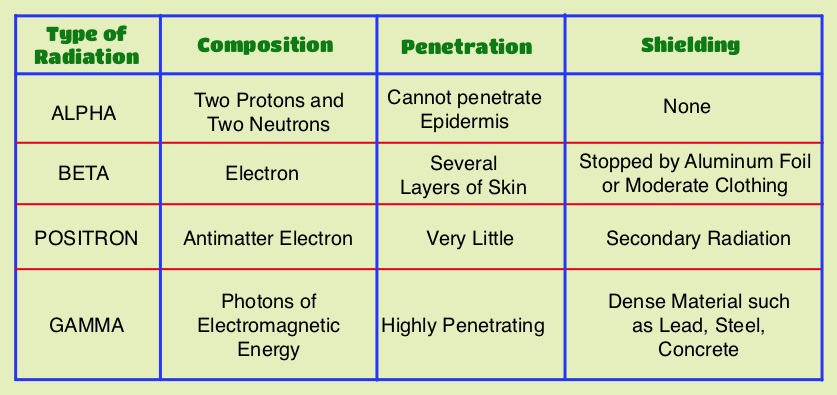 The primary endpoint was Overall Survival and secondary endpoints included time to first symptomatic skeletal event, time to increase in total alkaline phosphatase level and PSA level. This study was stopped earlier than planned, as there was a significant increase in the median overall survival in the XOFIGO® group compared to placebo group, with a 30% reduction in the risk of death (14.9 months vs 11.3 months, HR=0.70, P<0.001). All secondary endpoints favored XOFIGO® as well. The most common adverse events were cytopenias, majority of which were Grades 1 and 2 and discontinuation rates due to adverse events were lower in the XOFIGO® group (17%) compared to 21% for the Placebo group. At 1.5 years, the incidence of myelosuppression was 3%, there were no grade 3 or 4 non-hematologic toxicities and no reports of secondary malignancies in the XOFIGO® group. The authors concluded that based on the long term safety data, XOFIGO® has an excellent side-effect profile in patients with CRPC. Unlike the bone seeking beta emitters such as Strontium-89 and Samarium-153, XOFIGO®, an alpha emitter, is the only agent that has been shown to improve overall survival. Studies are underway evaluating the efficacy of chemotherapy in combination with XOFIGO®, in patients with CRPC and associated bone metastases. Nilsson S, Vogelzang NJ, Sartor AO, et al. J Clin Oncol 32, 2014 (suppl 4; abstr 9)
The primary endpoint was Overall Survival and secondary endpoints included time to first symptomatic skeletal event, time to increase in total alkaline phosphatase level and PSA level. This study was stopped earlier than planned, as there was a significant increase in the median overall survival in the XOFIGO® group compared to placebo group, with a 30% reduction in the risk of death (14.9 months vs 11.3 months, HR=0.70, P<0.001). All secondary endpoints favored XOFIGO® as well. The most common adverse events were cytopenias, majority of which were Grades 1 and 2 and discontinuation rates due to adverse events were lower in the XOFIGO® group (17%) compared to 21% for the Placebo group. At 1.5 years, the incidence of myelosuppression was 3%, there were no grade 3 or 4 non-hematologic toxicities and no reports of secondary malignancies in the XOFIGO® group. The authors concluded that based on the long term safety data, XOFIGO® has an excellent side-effect profile in patients with CRPC. Unlike the bone seeking beta emitters such as Strontium-89 and Samarium-153, XOFIGO®, an alpha emitter, is the only agent that has been shown to improve overall survival. Studies are underway evaluating the efficacy of chemotherapy in combination with XOFIGO®, in patients with CRPC and associated bone metastases. Nilsson S, Vogelzang NJ, Sartor AO, et al. J Clin Oncol 32, 2014 (suppl 4; abstr 9)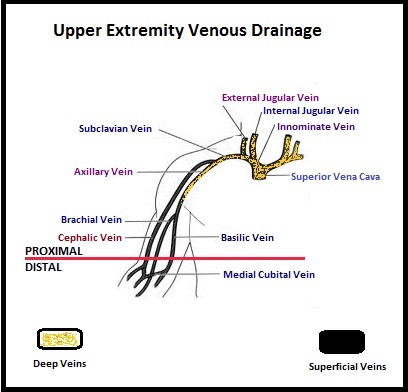 The authors characterized the NLDVT as Prevalent or Incident (depending on whether the thrombosis was identified within 72 hours of ICU admission or developed after the third ICU day) and whether they were catheter related or not. Risk factors for NLDVT and subsequent anticoagulant therapy, associated PE, and death were evaluated. Several important findings were noted from this study. Of the 3746 patients, 2.2% developed 1 or more Non-Leg Vein Thromboses (superficial or deep, proximal or distal). Majority of these thrombotic events (95%) occurred in the upper extremity and most of these occurred in the clinically important proximal and deep venous system. Further, these thrombotic episodes were more commonly Incident (2.0%) rather than Prevalent (0.2%) – P <0.001. It was noted that 1 in 7 patients with NLDVT developed PE. It appears that malignancy, hospitalization and Central Venous Catheters are risk factors for Upper Extremity Deep Vein Thrombosis (UEDVT), but the disproportionate increase in the incidence of UEDVT in hospitalized patients (30%-40% of hospital associated DVT’s) has been attributed to the increased use of CVC’s such as PICC (Peripherally Inserted Central Catheter). In this study, only 13% of the patients diagnosed with NLDVT received anticoagulation therapy. In an accompanying commentary by Dr. Greg Maynard, it was well pointed out that UEDVT is associated with similar rates of recurrence, PE and mortality as lower extremity DVT and as such UEDVT should be treated with anticoagulant therapy similar to Lower Extremity DVT. Dr. Maynard also noted that PICC associated DVT could be significantly reduced by appropriately choosing patients for a PICC line, proper PICC placement, early PICC removal, smaller diameter PICC and smaller number of lumens in the catheter. The authors concluded that despite universal prophylaxis with heparin, there was a high incidence of NLDVT in clinically important locations of the venous system, in critically ill patients and this calls for more structured preventive measures, as we learn more about this entity. Lamontagne F, McIntyre L, Dodek P, et al. JAMA Intern Med. 2014;174:689-696
The authors characterized the NLDVT as Prevalent or Incident (depending on whether the thrombosis was identified within 72 hours of ICU admission or developed after the third ICU day) and whether they were catheter related or not. Risk factors for NLDVT and subsequent anticoagulant therapy, associated PE, and death were evaluated. Several important findings were noted from this study. Of the 3746 patients, 2.2% developed 1 or more Non-Leg Vein Thromboses (superficial or deep, proximal or distal). Majority of these thrombotic events (95%) occurred in the upper extremity and most of these occurred in the clinically important proximal and deep venous system. Further, these thrombotic episodes were more commonly Incident (2.0%) rather than Prevalent (0.2%) – P <0.001. It was noted that 1 in 7 patients with NLDVT developed PE. It appears that malignancy, hospitalization and Central Venous Catheters are risk factors for Upper Extremity Deep Vein Thrombosis (UEDVT), but the disproportionate increase in the incidence of UEDVT in hospitalized patients (30%-40% of hospital associated DVT’s) has been attributed to the increased use of CVC’s such as PICC (Peripherally Inserted Central Catheter). In this study, only 13% of the patients diagnosed with NLDVT received anticoagulation therapy. In an accompanying commentary by Dr. Greg Maynard, it was well pointed out that UEDVT is associated with similar rates of recurrence, PE and mortality as lower extremity DVT and as such UEDVT should be treated with anticoagulant therapy similar to Lower Extremity DVT. Dr. Maynard also noted that PICC associated DVT could be significantly reduced by appropriately choosing patients for a PICC line, proper PICC placement, early PICC removal, smaller diameter PICC and smaller number of lumens in the catheter. The authors concluded that despite universal prophylaxis with heparin, there was a high incidence of NLDVT in clinically important locations of the venous system, in critically ill patients and this calls for more structured preventive measures, as we learn more about this entity. Lamontagne F, McIntyre L, Dodek P, et al. JAMA Intern Med. 2014;174:689-696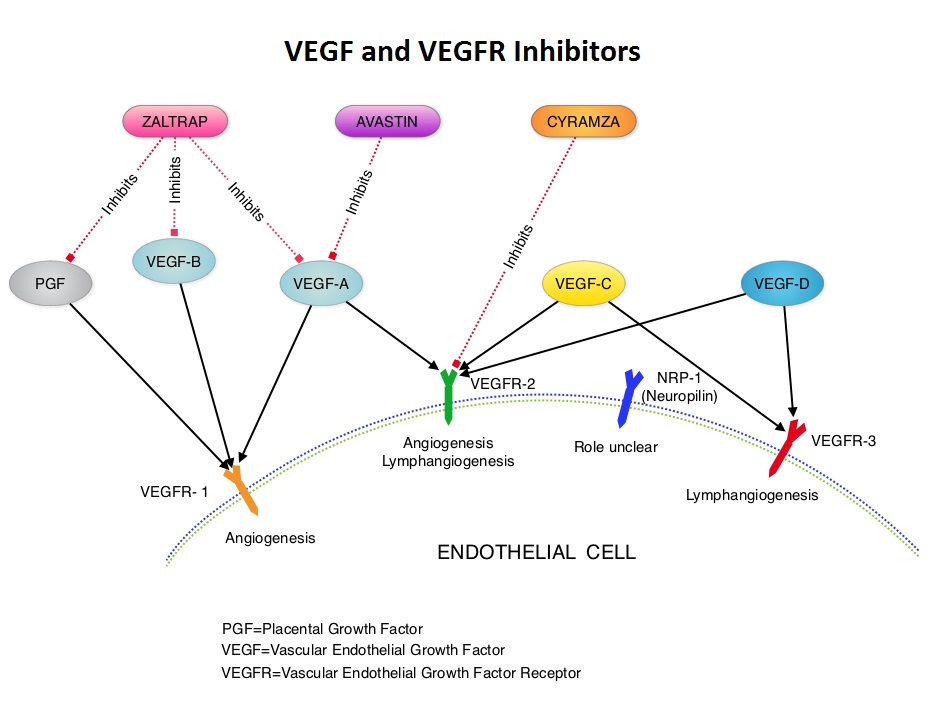 AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308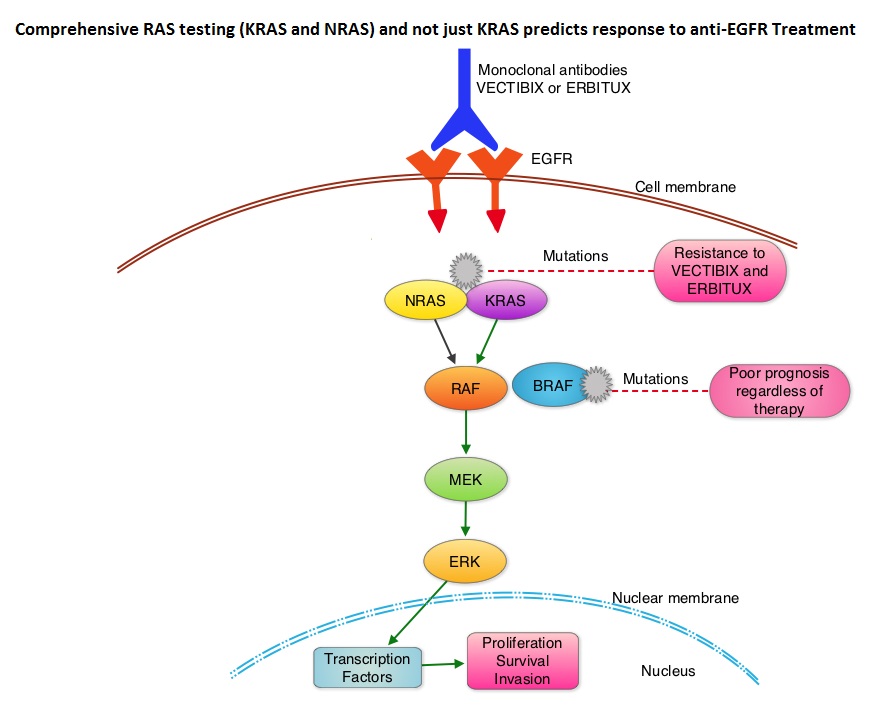 The most common RAS oncogenes in human cancer are HRAS, KRAS, and NRAS. Mutations in HRAS are not common in colon cancer whereas KRAS and NRAS mutations are seen in colon cancer and tend to be mutually exclusive. Activating mutations in exon 2 of the KRAS gene is seen in about 40% of colon cancer patients and predicts resistance to EGFR therapy. Mutational analysis is therefore usually performed to look for mutations in exon 2 of the KRAS gene. It appears however that a broader assessment of the RAS genes may more accurately predict resistance to EGFR therapy. It is also known that mutations in BRAF gene, which is downstream from RAS, may confer poor prognosis in colon cancer, regardless of therapy. The authors in a previous publication showed that in a phase III study involving 1,186 patients, the addition of VECTIBIX®, a fully human, EGFR targeted, monoclonal antibody, when combined with FOLFIRI (Folinic acid, Fluorouracil and Irinotecan), significantly improved Progression Free Survival, when compared to FOLFIRI alone (HR=0.73; P=0.004), with a trend towards improved Overall Survival (HR=0.85; P=0.12). Evolving data has suggested that testing for additional mutations in the RAS genes may help better understand the efficacy of/resistance to VECTIBIX®. In this present analysis, tumor samples of patients from the authors previous study, that were already known to be Wild Type KRAS – unmutated at KRAS exon 2 (N=597), were assessed for additional RAS mutations, specifically in KRAS exons 3 and 4 and NRAS exons 2, 3 and 4. Eighteen percent (18%) of the Wild Type KRAS exon 2 patients harbored additional RAS mutations. It was noted that patients receiving VECTIBIX® along with FOLFIRI had an improvement in both the median OS and PFS when they had wild-type (unmutated) RAS tumors compared to those, whose tumors harbored RAS mutations (median OS: 16.2 vs 11.8 months; median PFS: 6.4 vs 4.8 months). More importantly, amongst patients with mutated RAS tumors, the addition of VECTIBIX® to FOLFIRI resulted in no significant survival benefit compared to FOLFIRI alone (median OS: 11.8 vs 11.1 months; median PFS: 4.8 vs 4.0 months). The authors concluded that comprehensive RAS mutational analysis rather than KRAS testing alone, gives more relevant information for proper selection of patients with metastatic colorectal cancer, who would benefit from EGFR targeted monoclonal antibodies such as VECTIBIX®. In addition patients with RAS mutations could be spared from the associated cost and toxicites of EGFR targeted monoclonal antibodies that will not improve their outcomes. Peeters M, Oliner KS, Price TJ, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA387)
The most common RAS oncogenes in human cancer are HRAS, KRAS, and NRAS. Mutations in HRAS are not common in colon cancer whereas KRAS and NRAS mutations are seen in colon cancer and tend to be mutually exclusive. Activating mutations in exon 2 of the KRAS gene is seen in about 40% of colon cancer patients and predicts resistance to EGFR therapy. Mutational analysis is therefore usually performed to look for mutations in exon 2 of the KRAS gene. It appears however that a broader assessment of the RAS genes may more accurately predict resistance to EGFR therapy. It is also known that mutations in BRAF gene, which is downstream from RAS, may confer poor prognosis in colon cancer, regardless of therapy. The authors in a previous publication showed that in a phase III study involving 1,186 patients, the addition of VECTIBIX®, a fully human, EGFR targeted, monoclonal antibody, when combined with FOLFIRI (Folinic acid, Fluorouracil and Irinotecan), significantly improved Progression Free Survival, when compared to FOLFIRI alone (HR=0.73; P=0.004), with a trend towards improved Overall Survival (HR=0.85; P=0.12). Evolving data has suggested that testing for additional mutations in the RAS genes may help better understand the efficacy of/resistance to VECTIBIX®. In this present analysis, tumor samples of patients from the authors previous study, that were already known to be Wild Type KRAS – unmutated at KRAS exon 2 (N=597), were assessed for additional RAS mutations, specifically in KRAS exons 3 and 4 and NRAS exons 2, 3 and 4. Eighteen percent (18%) of the Wild Type KRAS exon 2 patients harbored additional RAS mutations. It was noted that patients receiving VECTIBIX® along with FOLFIRI had an improvement in both the median OS and PFS when they had wild-type (unmutated) RAS tumors compared to those, whose tumors harbored RAS mutations (median OS: 16.2 vs 11.8 months; median PFS: 6.4 vs 4.8 months). More importantly, amongst patients with mutated RAS tumors, the addition of VECTIBIX® to FOLFIRI resulted in no significant survival benefit compared to FOLFIRI alone (median OS: 11.8 vs 11.1 months; median PFS: 4.8 vs 4.0 months). The authors concluded that comprehensive RAS mutational analysis rather than KRAS testing alone, gives more relevant information for proper selection of patients with metastatic colorectal cancer, who would benefit from EGFR targeted monoclonal antibodies such as VECTIBIX®. In addition patients with RAS mutations could be spared from the associated cost and toxicites of EGFR targeted monoclonal antibodies that will not improve their outcomes. Peeters M, Oliner KS, Price TJ, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA387)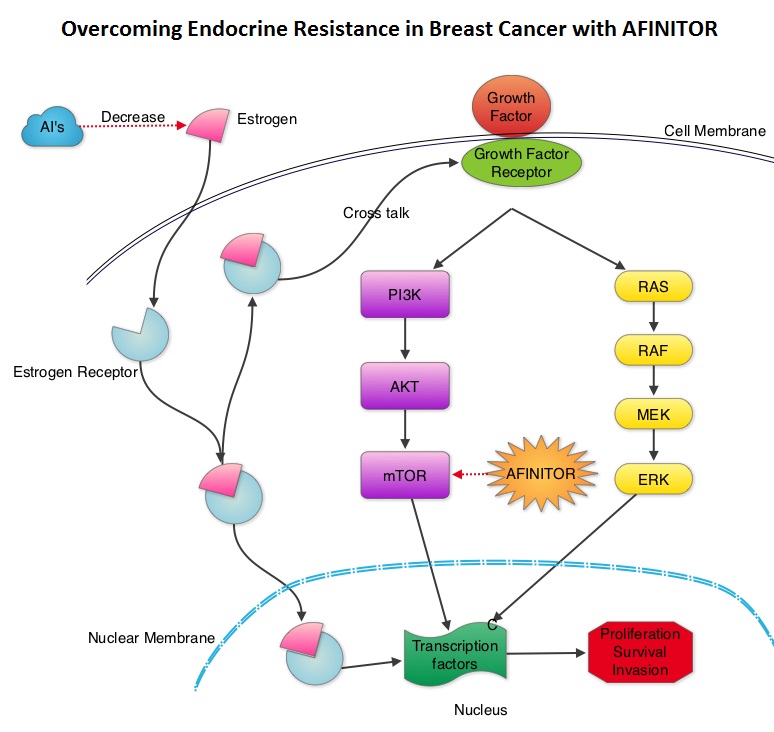 The mechanism of endocrine resistance has been attributed to cross talk between Estrogen Receptor signaling and PI3K/AKT/mTOR pathway. The PI3K/AKT/mTOR is a complex pathway, essential for cell proliferation, survival and apoptosis (programmed cell death). This pathway is of interest because of its increased activity in malignant cells as a result of amplification or mutation of the genes associated with PI3-kinase (phosphatidylinositol-3 kinase) and AKT, as well as loss of function of PTEN. PTEN normally prevents activation of AKT and its downstream pathways. Therefore, elevated ER signaling and breast cancer progression has been associated with activation of this mTOR (mammalian Target Of Rapamycin) pathway. Everolimus (AFINITOR®) is a mTOR inhibitor that has been shown to inhibit ER signaling and restore sensitivity to anti-estrogen therapies. With this preclinical knowledge, the Breast cancer trial of OraL EveROlimus-2 (BOLERO-2), which is a randomized, multicenter phase III trial was conducted to evaluate the benefit of combining steroidal AI, AROMASIN® (Exemestane) and an mTOR inhibitor AFINITOR®, for treatment of postmenopausal patients with HR+ and HER2 negative advanced breast cancer, who had either recurrent or progressive disease after non steroidal AI’s. Seven hundred and twenty four (N=724) patients were randomly assigned in a 2:1 ratio to receive either a combination of a steroidal AI, AROMASIN® at 25 mg PO QD and AFINITOR® at 10 mg PO QD (N=485) or AROMASIN® along with a placebo (N=239). Both treatment groups were well balanced and patients were stratified according to sensitivity to previous hormonal therapy and presence of visceral metastases. The primary endpoint for this study was Progression Free Survival (PFS) and secondary endpoints included overall Response Rate (RR), Clinical Benefit Rate (CBR defined as complete response + partial response + stable disease for at least 6 months), Overall Survival, Quality of Life, changes in bone marker levels and patient safety. In this study, close to 60% of the patients had visceral disease and approximately 80% of the patients had prior therapies for metastatic disease, with only 20% receiving the study drugs as their first therapy for metastatic disease. The combination of AFINITOR® and AROMASIN® significantly prolonged PFS compared to AROMASIN® alone (11 vs 4.1 months, HR=0.38, P<0.0001) and the Clinical Benefit Rate was 51% in the combination group and 26% in the AROMASIN® alone group (P<0.0001). The combination of AFINITOR® and AROMASIN® benefitted all subgroups of patients including those who had disease recurrence during or after neoadjuvant/adjuvant non steroidal AI therapy, those with visceral and bone metastases, as well as those who had prior chemotherapy. The most common adverse events in all age groups were rash, stomatitis, fatigue, diarrhea and nausea and these toxicities were manageable. The authors concluded that AFINITOR® given along with AROMASIN® in the study population can decrease the risk of disease progression by 60% and can double the Clinical Benefit Rate, compared to AROMASIN® alone, even in patients 65 years of age or older, thereby delaying the need for chemotherapy. Beck JT, Hortobagyi GN, Campone M, et al. Breast Cancer Res Treat. 2014; 143: 459–467
The mechanism of endocrine resistance has been attributed to cross talk between Estrogen Receptor signaling and PI3K/AKT/mTOR pathway. The PI3K/AKT/mTOR is a complex pathway, essential for cell proliferation, survival and apoptosis (programmed cell death). This pathway is of interest because of its increased activity in malignant cells as a result of amplification or mutation of the genes associated with PI3-kinase (phosphatidylinositol-3 kinase) and AKT, as well as loss of function of PTEN. PTEN normally prevents activation of AKT and its downstream pathways. Therefore, elevated ER signaling and breast cancer progression has been associated with activation of this mTOR (mammalian Target Of Rapamycin) pathway. Everolimus (AFINITOR®) is a mTOR inhibitor that has been shown to inhibit ER signaling and restore sensitivity to anti-estrogen therapies. With this preclinical knowledge, the Breast cancer trial of OraL EveROlimus-2 (BOLERO-2), which is a randomized, multicenter phase III trial was conducted to evaluate the benefit of combining steroidal AI, AROMASIN® (Exemestane) and an mTOR inhibitor AFINITOR®, for treatment of postmenopausal patients with HR+ and HER2 negative advanced breast cancer, who had either recurrent or progressive disease after non steroidal AI’s. Seven hundred and twenty four (N=724) patients were randomly assigned in a 2:1 ratio to receive either a combination of a steroidal AI, AROMASIN® at 25 mg PO QD and AFINITOR® at 10 mg PO QD (N=485) or AROMASIN® along with a placebo (N=239). Both treatment groups were well balanced and patients were stratified according to sensitivity to previous hormonal therapy and presence of visceral metastases. The primary endpoint for this study was Progression Free Survival (PFS) and secondary endpoints included overall Response Rate (RR), Clinical Benefit Rate (CBR defined as complete response + partial response + stable disease for at least 6 months), Overall Survival, Quality of Life, changes in bone marker levels and patient safety. In this study, close to 60% of the patients had visceral disease and approximately 80% of the patients had prior therapies for metastatic disease, with only 20% receiving the study drugs as their first therapy for metastatic disease. The combination of AFINITOR® and AROMASIN® significantly prolonged PFS compared to AROMASIN® alone (11 vs 4.1 months, HR=0.38, P<0.0001) and the Clinical Benefit Rate was 51% in the combination group and 26% in the AROMASIN® alone group (P<0.0001). The combination of AFINITOR® and AROMASIN® benefitted all subgroups of patients including those who had disease recurrence during or after neoadjuvant/adjuvant non steroidal AI therapy, those with visceral and bone metastases, as well as those who had prior chemotherapy. The most common adverse events in all age groups were rash, stomatitis, fatigue, diarrhea and nausea and these toxicities were manageable. The authors concluded that AFINITOR® given along with AROMASIN® in the study population can decrease the risk of disease progression by 60% and can double the Clinical Benefit Rate, compared to AROMASIN® alone, even in patients 65 years of age or older, thereby delaying the need for chemotherapy. Beck JT, Hortobagyi GN, Campone M, et al. Breast Cancer Res Treat. 2014; 143: 459–467