SUMMARY: The American Cancer Society estimates that approximately 137,000 new cases of colorectal cancer will be diagnosed in the United States in 2014 and over 50,000 are expected to die of the disease. Data from 60 epidemiological studies enrolling more than 26,000 ColoRectal Cancer (CRC) patients have shown that higher consumption of milk and dairy products reduces the risk of colon cancer and high Calcium intake reduces the risk of CRC. In vivo and in vitro studies have confirmed these findings.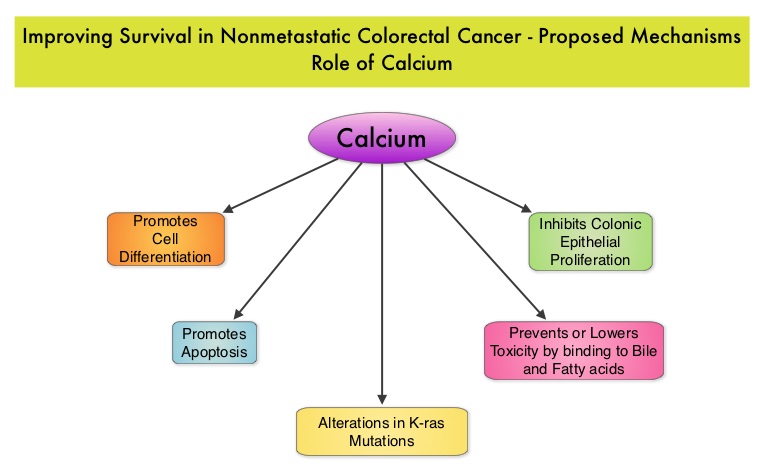 Milk, in addition to being a rich source of dietary Calcium and Vitamin D is a primary dietary source of conjugated linoleic acid which has been shown to inhibit colon cancer cell growth. Dairy products also provide other beneficial components such as butyric acid, lactoferrin and fermentation products. The impact of milk and dairy products on CRC survival however has remained unclear. The Cancer Prevention Study – II Nutrition Cohort is a prospective study of cancer incidence that began in 1992. Participants in this study (N=184,000) were provided a self administered questionnaire and baseline information about their dietary habits (including dietary Calcium and Vitamin D, as well as Calcium, Vitamin D and multivitamin supplements), physical activity, body size, cancer screening and early detection, etc. was collected and follow up questionnaires were sent every other year to update information and learn about new cancer diagnosis. Patients were followed up until June 2009 and by the end of this period, 3,832 individuals who had no history of disease at baseline had been diagnosed with invasive colon or rectal cancer. After excluding patients with distant metastatic disease, 2,284 patients were included in this analysis and among them, 1,111 patients reported post diagnosis diet. The primary outcome of this study was all cause mortality and the secondary outcome was mortality resulting from colorectal cancer. Using standard statistical models, the investigators noted that post CRC diagnosis total Calcium intake and milk intake, was inversely associated with all-cause mortality and significantly reduced CRC specific mortality.
Milk, in addition to being a rich source of dietary Calcium and Vitamin D is a primary dietary source of conjugated linoleic acid which has been shown to inhibit colon cancer cell growth. Dairy products also provide other beneficial components such as butyric acid, lactoferrin and fermentation products. The impact of milk and dairy products on CRC survival however has remained unclear. The Cancer Prevention Study – II Nutrition Cohort is a prospective study of cancer incidence that began in 1992. Participants in this study (N=184,000) were provided a self administered questionnaire and baseline information about their dietary habits (including dietary Calcium and Vitamin D, as well as Calcium, Vitamin D and multivitamin supplements), physical activity, body size, cancer screening and early detection, etc. was collected and follow up questionnaires were sent every other year to update information and learn about new cancer diagnosis. Patients were followed up until June 2009 and by the end of this period, 3,832 individuals who had no history of disease at baseline had been diagnosed with invasive colon or rectal cancer. After excluding patients with distant metastatic disease, 2,284 patients were included in this analysis and among them, 1,111 patients reported post diagnosis diet. The primary outcome of this study was all cause mortality and the secondary outcome was mortality resulting from colorectal cancer. Using standard statistical models, the investigators noted that post CRC diagnosis total Calcium intake and milk intake, was inversely associated with all-cause mortality and significantly reduced CRC specific mortality.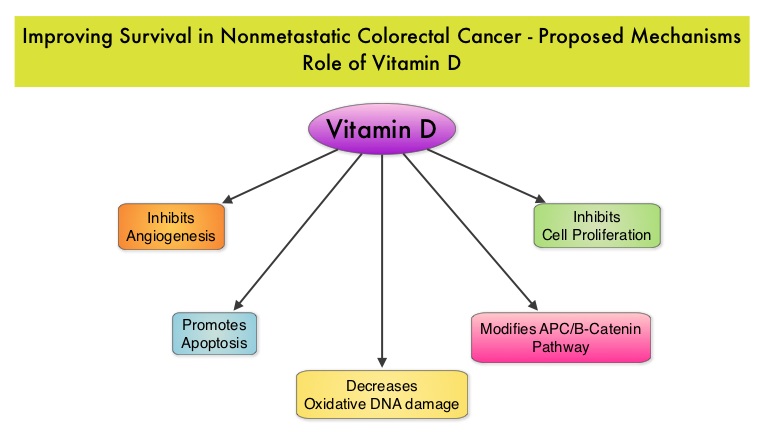 This benefit however, was not seen with Vitamin D intake. Also of interest, pre-diagnosis Calcium, Vitamin D, and dairy product intakes did not influence mortality outcomes. The authors concluded that higher post-diagnosis intakes of total Calcium and milk may be associated with lower risk of death among patients with non-metastatic ColoRectal Cancer. In a more recent publication, it has been reported that there is a strong association between plasma level of 25-hydroxyvitamin D (25-OHD) and CRC specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25-OHD (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). With 30-35% of the malignancies attributed to dietary habits, the onus is therefore on the treating physicians to provide nutrition counseling during and after cancer treatment and is not to be ignored. Yang B, McCullough ML, Gapstur SM, et al. J Clin Oncol 2014;32:2335-2343
This benefit however, was not seen with Vitamin D intake. Also of interest, pre-diagnosis Calcium, Vitamin D, and dairy product intakes did not influence mortality outcomes. The authors concluded that higher post-diagnosis intakes of total Calcium and milk may be associated with lower risk of death among patients with non-metastatic ColoRectal Cancer. In a more recent publication, it has been reported that there is a strong association between plasma level of 25-hydroxyvitamin D (25-OHD) and CRC specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25-OHD (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). With 30-35% of the malignancies attributed to dietary habits, the onus is therefore on the treating physicians to provide nutrition counseling during and after cancer treatment and is not to be ignored. Yang B, McCullough ML, Gapstur SM, et al. J Clin Oncol 2014;32:2335-2343
Month: August 2014
Randomized, Controlled, Double-Blind, Cross-Over Trial Assessing Treatment Preference for Pazopanib Versus Sunitinib in Patients With Metastatic Renal Cell Carcinoma PISCES Study
SUMMARY: Kidney cancer is among the 10 most common cancers in both men and women and the American Cancer Society estimates that approximately 64,000 new cases of kidney cancer will be diagnosed for 2014 in the United States and about 13,860 individuals will die from this disease. Renal cell carcinoma is a highly vascular tumor. Vascular Endothelial Growth Factor (VEGF), PDGF (Platelet Derived Growth Factor) and FGF (Fibroblast Growth Factor) are the major growth factors involved in angiogenesis, with VEGF playing the most important role, regulating vascular permeability. These ligands (VEGF, PDGF and FGF) bind to their respective receptors which exhibit tyrosine kinase activity and are able to activate the signaling pathways down stream. The study of Von Hippel-Lindau (VHL) syndrome and the cloning of VHL gene (a tumor suppressor gene) led to the understanding of the molecular basis and biology of the most common type of sporadic Renal Cell Carcinoma (Clear Cell Carcinoma). All patients with VHL syndrome have a germ line inactivating mutation of one VHL allele and inactivation of the second allele is the triggering event for the formation of Clear Cell Carcinomas of the kidney, as well as other tumors including endocrine Pancreatic tumors, Papillary cystadenomas of the pancreas, adnexal organs, epididymis, endolymphatic system of the inner ear, Hemangioblastoma of the CNS and retina and Pheochromocytomas. It appears that close to 60% of the patients with non-hereditary (sporadic) clear cell carcinoma of the kidney have loss of function of the VHL gene.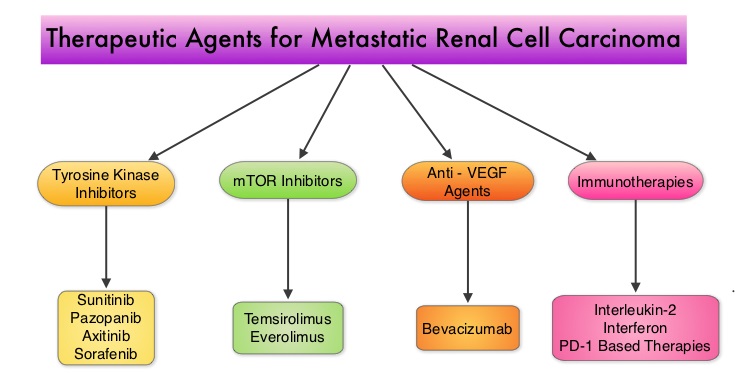 The understanding of the biology of clear cell carcinoma of the kidney has led to the development of multitargeted Tyrosine Kinase Inhibitors, which are small molecules that simultaneously target the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/ smooth vessel cell wall. The end result is decreased angiogenesis, cell proliferation and cell survival of both endothelial cells as well as tumor cells. There are four classes of agents presently available for the treatment of metastatic Renal Cell carcinoma. They include Immunotherapies, Anti-VEGF agents, Tyrosine Kinase Inhibitors (TKI’s) and mTOR (Mammalian Target of Rapamycin) inhibitors. The choice of First Line therapies for metastatic Renal Cell Carcinoma is based on MSKCC (Memorial Sloan Kettering Cancer Center) risk classification and the agents include, Temsirolimus (TORISEL®) for poor risk patients and TKI’s as well as Immunotherapy with or without anti-VEGF agents for good and intermediate risk patients.
The understanding of the biology of clear cell carcinoma of the kidney has led to the development of multitargeted Tyrosine Kinase Inhibitors, which are small molecules that simultaneously target the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/ smooth vessel cell wall. The end result is decreased angiogenesis, cell proliferation and cell survival of both endothelial cells as well as tumor cells. There are four classes of agents presently available for the treatment of metastatic Renal Cell carcinoma. They include Immunotherapies, Anti-VEGF agents, Tyrosine Kinase Inhibitors (TKI’s) and mTOR (Mammalian Target of Rapamycin) inhibitors. The choice of First Line therapies for metastatic Renal Cell Carcinoma is based on MSKCC (Memorial Sloan Kettering Cancer Center) risk classification and the agents include, Temsirolimus (TORISEL®) for poor risk patients and TKI’s as well as Immunotherapy with or without anti-VEGF agents for good and intermediate risk patients. 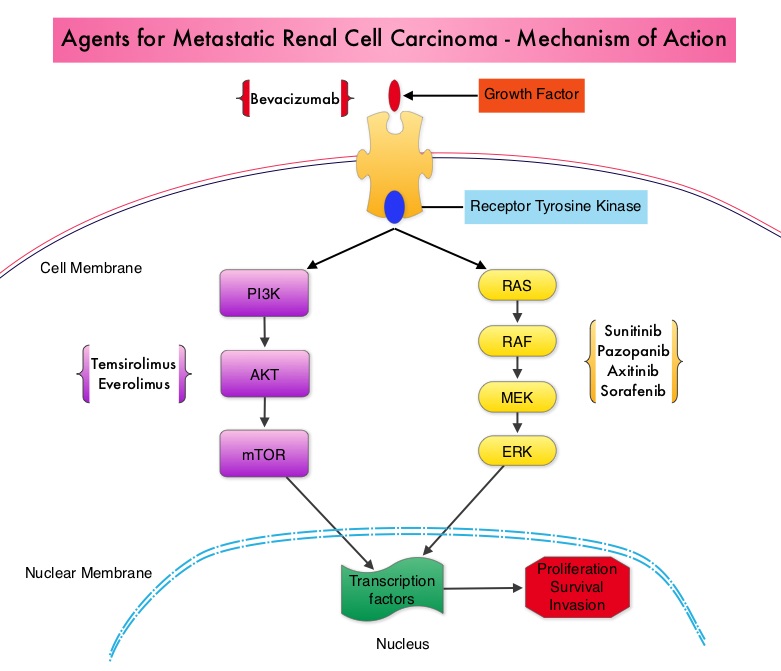 The TKI’s of choice however for good to intermediate risk mRCC patients with clear cell histology are, either SUTENT® (Sunitinib) or VOTRIENT® (Pazopanib). Even though both agents have similar efficacy, it remained unclear if one agent was better tolerated than the other. To address this, the authors evaluated patient preference for VOTRIENT® or SUTENT® in a double blind, phase III cross over study. The primary end point of this study was patient preference for a specific treatment, as assessed by a questionnaire at the end of the two treatment periods. Other endpoints included reasons for preference, physician preference, safety, and HRQoL (Health Related Quality of Life). Randomly assigned patients with metastatic Renal Cell Carcinoma (mRCC) with clear cell histology (N=168), received VOTRIENT® 800 mg per day for 10 weeks followed by a 2 week washout period and then SUTENT® 50 mg per day (4 weeks on, 2 weeks off, 4 weeks on) for 10 weeks (N=86), or the reverse sequence (N=82). One hundred and fourteen (N=114) patients met the prespecified modified intent-to-treat criteria for the primary analysis and the criteria included exposure to both of the treatments, no disease progression before cross over and completion of the preference questionnaire. When outcomes were evaluated, 70% of the patients preferred VOTRIENT®, 22% preferred SUTENT® and 8% had no preference (P<0.001).
The TKI’s of choice however for good to intermediate risk mRCC patients with clear cell histology are, either SUTENT® (Sunitinib) or VOTRIENT® (Pazopanib). Even though both agents have similar efficacy, it remained unclear if one agent was better tolerated than the other. To address this, the authors evaluated patient preference for VOTRIENT® or SUTENT® in a double blind, phase III cross over study. The primary end point of this study was patient preference for a specific treatment, as assessed by a questionnaire at the end of the two treatment periods. Other endpoints included reasons for preference, physician preference, safety, and HRQoL (Health Related Quality of Life). Randomly assigned patients with metastatic Renal Cell Carcinoma (mRCC) with clear cell histology (N=168), received VOTRIENT® 800 mg per day for 10 weeks followed by a 2 week washout period and then SUTENT® 50 mg per day (4 weeks on, 2 weeks off, 4 weeks on) for 10 weeks (N=86), or the reverse sequence (N=82). One hundred and fourteen (N=114) patients met the prespecified modified intent-to-treat criteria for the primary analysis and the criteria included exposure to both of the treatments, no disease progression before cross over and completion of the preference questionnaire. When outcomes were evaluated, 70% of the patients preferred VOTRIENT®, 22% preferred SUTENT® and 8% had no preference (P<0.001). 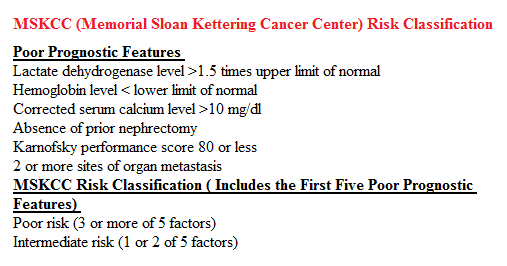 The main reasons for preferring VOTRIENT® were less fatigue and better overall quality of life whereas those who preferred SUTENT® cited less diarrhea as the main reason. Physician preference was also taken into consideration in this study, as physicians are able to better assess efficacy and asymptomatic toxicities that are clinically relevant. VOTRIENT® was preferred by 61% of the physicians, 22% preferred SUTENT® and 17% had no preference. VOTRIENT® was also superior to SUTENT® with regards to HRQoL measures that evaluated fatigue, hand/foot soreness and mouth/throat soreness. The authors concluded that this innovative cross-over trial demonstrated that significantly more patients preferred VOTRIENT® over SUTENT®, based on lower rates of adverse events and better Health-Related Quality of Life. Escudier B, Porta C, Bono P, et al. J Clin Oncol 2014;32:1412-1418
The main reasons for preferring VOTRIENT® were less fatigue and better overall quality of life whereas those who preferred SUTENT® cited less diarrhea as the main reason. Physician preference was also taken into consideration in this study, as physicians are able to better assess efficacy and asymptomatic toxicities that are clinically relevant. VOTRIENT® was preferred by 61% of the physicians, 22% preferred SUTENT® and 17% had no preference. VOTRIENT® was also superior to SUTENT® with regards to HRQoL measures that evaluated fatigue, hand/foot soreness and mouth/throat soreness. The authors concluded that this innovative cross-over trial demonstrated that significantly more patients preferred VOTRIENT® over SUTENT®, based on lower rates of adverse events and better Health-Related Quality of Life. Escudier B, Porta C, Bono P, et al. J Clin Oncol 2014;32:1412-1418
ZYDELIG® – A New Treatment Option for Non-Hodgkin Lymphoma
The FDA granted ZYDELIG®, accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma. The approval was based on ZYDELIG®’s significant single agent activity with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. ZYDELIG® is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. More information is available at www.oncoprescribe.com
Phase III trial (Prevention of Early Menopause Study [POEMS]-SWOG S0230) of LHRH analog during chemotherapy (CT) to reduce ovarian failure in early-stage, hormone receptor-negative breast cancer An international Intergroup trial of SWOG, IBCSG, ECOG, and CALGB (Alliance)
SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive (Estrogen Receptor/Progesterone Receptor positive) and this is a predictor of response to endocrine therapy. In premenopausal woman, the ovary is the main source of estrogen production, whereas in postmenopausal women, the primary source of estrogen is the Aromatase enzyme mediated conversion of androstenedione and testosterone to estrone and estradiol in extragonadal/peripheral tissues. Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. 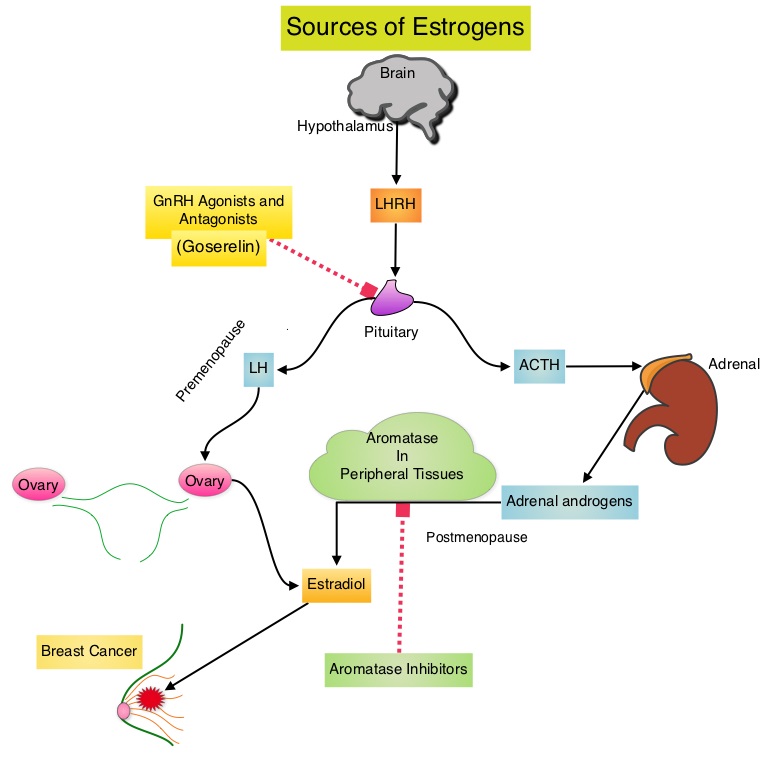 POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
PI3Kδ Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma
SUMMARY: Non-Hodgkin Lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. PI3K delta signaling is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. PI3K (PhosphatidylInositol 3-Kinase) is a lipid kinase and has four distinct isoforms – alpha, beta, gamma and delta. The alpha and beta isoforms are expressed in a wide variety of tissues whereas the gamma and delta isoforms are only expressed in hematopoietic cells. PI3K delta (the delta isoform of PI3K enzyme) is predominantly expressed in leukocytes and plays an important role in the normal B lymphocyte development as well as signal transduction from B cell receptor as well as receptors for various cytokines and chemokines.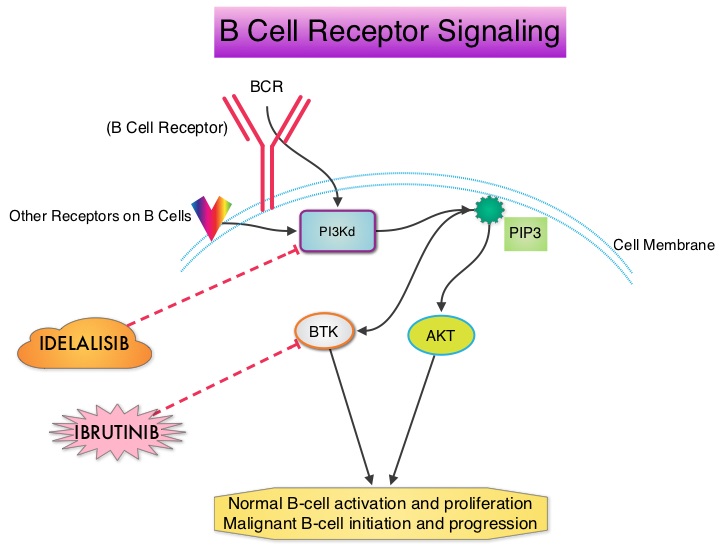 PI3K delta is activated by BCR signaling resulting in the production of a second messenger, Phosphatidylinositol 3,4,5-triphosphate (PIP3) which in turn activates Bruton’s Tyrosine Kinase (BTK) and AKT, a prosurvival kinase. Idelalisib (ZYDELIG®) is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. The FDA granted ZYDELIG® accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma based on the results of a single-arm, open-label, phase II trial. Patients with indolent Non-Hodgkin Lymphomas (N=125), who were refractory to Rituximab (RITUXAN®) and an alkylating agent or had relapsed within 6 months after receipt of these therapies, received ZYDELIG®, 150 mg PO BID. Treatment was continued until disease progression or unacceptable toxicities developed. The median age was 64 years and enrolled patients had received a median of four prior therapies. The indolent Non-Hodgkin Lymphoma subtypes included Follicular lymphoma (N=72), Small Lymphocytic Lymphoma (N=28), Marginal Zone Lymphoma (N=15), and Lymphoplasmacytic Lymphoma with or without Waldenström's Macroglobulinemia (N=10). The primary end point of this study was Overall Response Rate and secondary end points included the Duration of Response, Progression Free Survival, and Safety. The median follow up was 9.7 months. The Overall Response Rate was 57% with 50% partial responses and 6% complete responses. There was no difference in the Reponse Rates across the various subtypes of Indolent Non-Hodgkin Lymphomas. The median time to response was 1.9 months and the median duration of response was 12.5 months. The median Progression Free Survival was 11 months and the Overall Survival at one year was estimated to be 80%. The most common grade 3 or higher adverse events were diarrhea (13%), neutropenia (27%) and elevations in SGOT and SGPT levels (13%). These toxicities were manageable with dose modifications and dose interruptions. The authors concluded that ZYDELIG® has significant single agent activity, with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. Gopal AK, Kahl BS, de Vos S, et al. N Engl J Med 2014; 370:1008-1018
PI3K delta is activated by BCR signaling resulting in the production of a second messenger, Phosphatidylinositol 3,4,5-triphosphate (PIP3) which in turn activates Bruton’s Tyrosine Kinase (BTK) and AKT, a prosurvival kinase. Idelalisib (ZYDELIG®) is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. The FDA granted ZYDELIG® accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma based on the results of a single-arm, open-label, phase II trial. Patients with indolent Non-Hodgkin Lymphomas (N=125), who were refractory to Rituximab (RITUXAN®) and an alkylating agent or had relapsed within 6 months after receipt of these therapies, received ZYDELIG®, 150 mg PO BID. Treatment was continued until disease progression or unacceptable toxicities developed. The median age was 64 years and enrolled patients had received a median of four prior therapies. The indolent Non-Hodgkin Lymphoma subtypes included Follicular lymphoma (N=72), Small Lymphocytic Lymphoma (N=28), Marginal Zone Lymphoma (N=15), and Lymphoplasmacytic Lymphoma with or without Waldenström's Macroglobulinemia (N=10). The primary end point of this study was Overall Response Rate and secondary end points included the Duration of Response, Progression Free Survival, and Safety. The median follow up was 9.7 months. The Overall Response Rate was 57% with 50% partial responses and 6% complete responses. There was no difference in the Reponse Rates across the various subtypes of Indolent Non-Hodgkin Lymphomas. The median time to response was 1.9 months and the median duration of response was 12.5 months. The median Progression Free Survival was 11 months and the Overall Survival at one year was estimated to be 80%. The most common grade 3 or higher adverse events were diarrhea (13%), neutropenia (27%) and elevations in SGOT and SGPT levels (13%). These toxicities were manageable with dose modifications and dose interruptions. The authors concluded that ZYDELIG® has significant single agent activity, with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. Gopal AK, Kahl BS, de Vos S, et al. N Engl J Med 2014; 370:1008-1018
KADCYLA® beneficial for patients with HER2-positive Advanced Breast Cancer who had previously received HERCEPTIN® and TYKERB®.
KADCYLA® (Ado-Trastuzumab Emtansine, T-DM1) is an antibody-drug conjugate (ADC) comprised of the antibody HERCEPTIN® (Trastuzumab) and a chemotherapy agent Emtansine, linked together. Upon binding to the HER2 receptor, KADCYLA® not only inhibits the HER2 signaling pathways but also delivers Emtansine, a microtubule inhibitor, directly inside the tumor cells and destroys them. In the TH3RESA trial, treatment with KADCYLA® significantly improved Progression Free Survival compared to physicians choice, for those patients who had previously received HERCEPTIN® and TYKERB® (Lapatinib) and this therefore makes KADCYLA® the treatment of choice, for this patient population.
AVASTIN® (Bevacizumab)
The FDA on August 14, 2014 approved AVASTIN® for the treatment of patients with persistent, recurrent or metastatic Cervical Cancer, in combination with TAXOL® (Paclitaxel) and Cisplatin or TAXOL® and Topotecan (HYCAMTIN®). AVASTIN® is a product of Genentech, Inc.
Results of a prospective, randomized, open-label phase 3 study of ruxolitinib (RUX) in polycythemia vera (PV) patients resistant to or intolerant of hydroxyurea (HU) the RESPONSE trial
SUMMARY: Polycythemia Vera (P. Vera) is a clonal myeloproliferative neoplasm characterized by isolated Erythrocytosis in a majority of the patients, with the remaining demonstrating Erythrocytosis, Leukocytosis and/or Thrombocytosis along with Erythrocytosis. Patients usually present with this disorder in their sixth decade and are often asymptomatic, with the diagnosis made incidentally on routine laboratory evaluation. About 30% of the patients however, may initially present with a thrombotic episode, whereas a small percentage of patients may present with disease related symptoms such as pruritus and fatigue.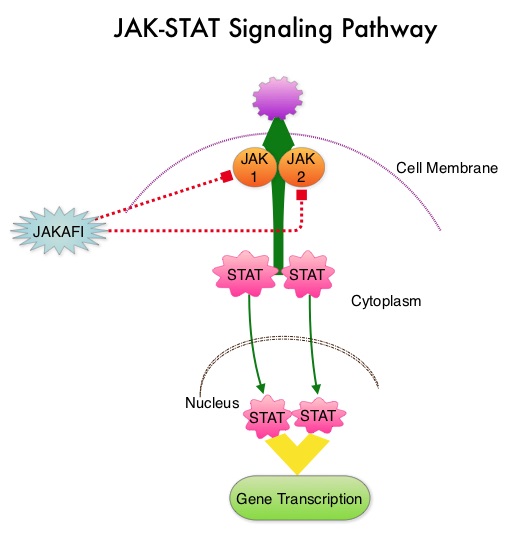 Over activation of the JAK-STAT signal transduction pathway caused by V617F mutation, has been implicated in majority of the patients with P. Vera. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with P. Vera, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. JAK2 mutations such as JAK2 V617F are seen in approximately 95% of patients with P. Vera. The goals of therapy in P. Vera are to maintain the hematocrit at less than 45% and decrease the risk of thrombosis and bleeding. P. Vera is presently managed with periodic phlebotomies, cytoreductive therapy with oral antimetabolite, Hydroxyurea and antiplatelet agents such as low dose aspirin. However, a significant number of patients on these therapies become intolerant or resistant to these treatments, leading to an increased risk of progression. JAKAFI® is a potent JAK1 and JAK2 inhibitor and exerts its mechanism of action by targeting and inhibiting the dysregulated JAK2-STAT signaling pathway.
Over activation of the JAK-STAT signal transduction pathway caused by V617F mutation, has been implicated in majority of the patients with P. Vera. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with P. Vera, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. JAK2 mutations such as JAK2 V617F are seen in approximately 95% of patients with P. Vera. The goals of therapy in P. Vera are to maintain the hematocrit at less than 45% and decrease the risk of thrombosis and bleeding. P. Vera is presently managed with periodic phlebotomies, cytoreductive therapy with oral antimetabolite, Hydroxyurea and antiplatelet agents such as low dose aspirin. However, a significant number of patients on these therapies become intolerant or resistant to these treatments, leading to an increased risk of progression. JAKAFI® is a potent JAK1 and JAK2 inhibitor and exerts its mechanism of action by targeting and inhibiting the dysregulated JAK2-STAT signaling pathway. 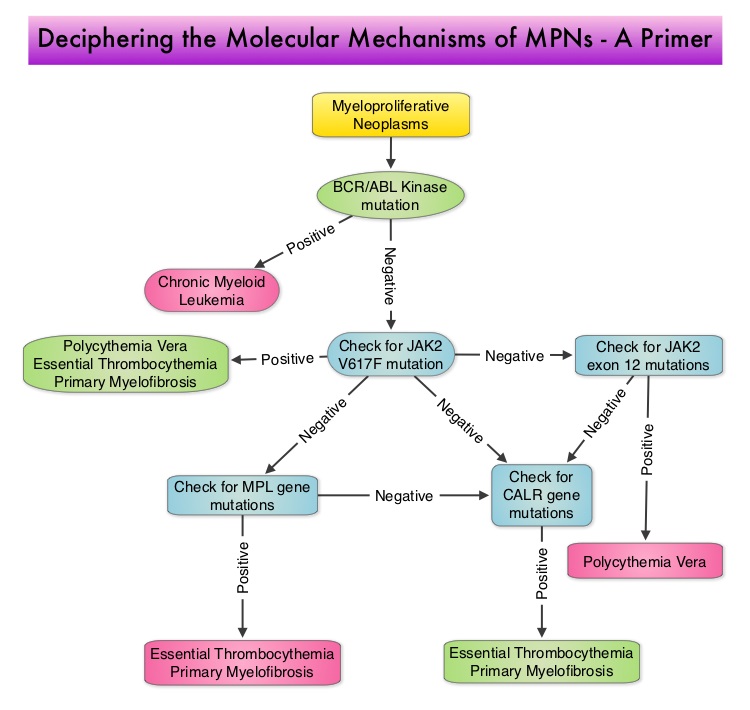 The RESPONSE trial is a phase III prospective randomized study in which patients with P. Vera, who were refractory or intolerant of Hydroxyurea were randomized to receive JAKAFI® 10 mg PO, bid (N=110) or Best Available Therapy (BAT), which consisted of investigator choice of monotherapy or observation only (N=112). Eligible patients were phlebotomy dependent patients with splenomegaly (> 450 cubic cm). Patients receiving BAT were allowed to cross over to JAKAFI® group from week 32 onwards. The primary endpoint of this study (composite primary endpoint) was the proportion of patients whose hematocrit was controlled without phlebotomy and whose spleen volume was reduced by 35% or more from baseline, as assessed by MRI imaging at 32 weeks. Secondary endpoints included durable response, Complete Hematological Remission and safety. The primary analysis was conducted when all patients reached week 48 or discontinued therapy. The proportion of patients in the JAKAFI® group who achieved the composite primary endpoint was 21% compared to 1% in the BAT group (P < 0.0001). Seventy seven percent (77%) of the patients in the JAKAFI® group achieved at least one of the two major components of the composite primary endpoint. Put another way, 60% of the patients in the JAKAFI® arm were able to achieve the target hematocrit level in the absence of phlebotomy, compared to 20% in the BAT group. Reduction in the spleen volume by 35% or more was noted in 38% of the patients in the JAKAFI® group compared to 1% in the BAT group. Complete Hematological Remission defined as continuous hematocrit below 45%, as well as normal white blood cells and platelets counts, was achieved in 24% and 9% of patients in JAKAFI® and BAT group respectively (P=0.003). More patients assigned to JAKAFI® group also demonstrated 50% or more improvement in the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) 14-item total symptom score, at week 32 compared to BAT (49% vs 5%). Thromboembolic events occurred in one patient assigned to the JAKAFI® group as compared to six patients in the BAT group. The authors concluded that JAKAFI® may represent a new option for treating high risk patients with Polycythemia Vera, who are refractory or intolerant of Hydroxyurea. Jakafi® is superior to Best Available Therapy (BAT) in controlling hematocrit without phlebotomies as well as Splenic Volume. Further, JAKAFI® is also effective in improving P. Vera associated symptoms. Verstovsek S, Kiladjian J, Griesshammer M, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7026)
The RESPONSE trial is a phase III prospective randomized study in which patients with P. Vera, who were refractory or intolerant of Hydroxyurea were randomized to receive JAKAFI® 10 mg PO, bid (N=110) or Best Available Therapy (BAT), which consisted of investigator choice of monotherapy or observation only (N=112). Eligible patients were phlebotomy dependent patients with splenomegaly (> 450 cubic cm). Patients receiving BAT were allowed to cross over to JAKAFI® group from week 32 onwards. The primary endpoint of this study (composite primary endpoint) was the proportion of patients whose hematocrit was controlled without phlebotomy and whose spleen volume was reduced by 35% or more from baseline, as assessed by MRI imaging at 32 weeks. Secondary endpoints included durable response, Complete Hematological Remission and safety. The primary analysis was conducted when all patients reached week 48 or discontinued therapy. The proportion of patients in the JAKAFI® group who achieved the composite primary endpoint was 21% compared to 1% in the BAT group (P < 0.0001). Seventy seven percent (77%) of the patients in the JAKAFI® group achieved at least one of the two major components of the composite primary endpoint. Put another way, 60% of the patients in the JAKAFI® arm were able to achieve the target hematocrit level in the absence of phlebotomy, compared to 20% in the BAT group. Reduction in the spleen volume by 35% or more was noted in 38% of the patients in the JAKAFI® group compared to 1% in the BAT group. Complete Hematological Remission defined as continuous hematocrit below 45%, as well as normal white blood cells and platelets counts, was achieved in 24% and 9% of patients in JAKAFI® and BAT group respectively (P=0.003). More patients assigned to JAKAFI® group also demonstrated 50% or more improvement in the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) 14-item total symptom score, at week 32 compared to BAT (49% vs 5%). Thromboembolic events occurred in one patient assigned to the JAKAFI® group as compared to six patients in the BAT group. The authors concluded that JAKAFI® may represent a new option for treating high risk patients with Polycythemia Vera, who are refractory or intolerant of Hydroxyurea. Jakafi® is superior to Best Available Therapy (BAT) in controlling hematocrit without phlebotomies as well as Splenic Volume. Further, JAKAFI® is also effective in improving P. Vera associated symptoms. Verstovsek S, Kiladjian J, Griesshammer M, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7026)
NCCN Guidelines for Survivorship Expanded to Address Two Common Conditions
SUMMARY: The National Comprehensive Cancer Network (NCCN) has expanded its Survivorship Guidelines to include cancer-associated cognitive impairment and Chemotherapy Induced Peripheral Neuropathy. The later is a component of the Adult Cancer Pain section. Dr. Urba discussed the management of Chemotherapy Induced Peripheral Neuropathy at the NCCN 19th annual conference. Approximately 20%-40% of the patients suffer from Chemotherapy Induced Peripheral Neuropathy, which can result in premature discontinuation of treatment. Further, this adverse event in a significant number of patients can persist indefinitely and can be disabling, thus impacting their activities of daily living. The following chemotherapeutic agents are associated with varying degrees of peripheral neuropathy – Platinum compounds (Cisplatin, Carboplatin and Oxaliplatin), Taxanes (Paclitaxel, Docetaxel), Immunomodulatory agents (Thalidomide, Lenalidomide), Other Microtubule inhibitors (Vincristine, Ixabepilone) and Proteosome Inhibitors (Bortezomib). It may be necessary to screen and rescreen patients for neuropathic pain, as patients may not be forthcoming with this complaint. Management of Neuropathic pain may include systemic treatment with adjuvant analgesics, topical therapies and psychosocial support. The management of Chemotherapy Induced Peripheral Neuropathy has mostly been extrapolated from validated studies on diabetic neuropathy. The first line treatment for Chemotherapy Induced Neuropathic Pain includes antidepressants and anticonvulsants, which if not effective on their own, can be combined with opioids. TriCyclic Antidepressants (TCA’s) such as Amitriptyline and Nortriptyline (PAMELOR®) can be considered as first line choice for appropriate patients, although its mechanism of action is uncertain and 20% of the patients discontinue therapy because of adverse effects.
The following chemotherapeutic agents are associated with varying degrees of peripheral neuropathy – Platinum compounds (Cisplatin, Carboplatin and Oxaliplatin), Taxanes (Paclitaxel, Docetaxel), Immunomodulatory agents (Thalidomide, Lenalidomide), Other Microtubule inhibitors (Vincristine, Ixabepilone) and Proteosome Inhibitors (Bortezomib). It may be necessary to screen and rescreen patients for neuropathic pain, as patients may not be forthcoming with this complaint. Management of Neuropathic pain may include systemic treatment with adjuvant analgesics, topical therapies and psychosocial support. The management of Chemotherapy Induced Peripheral Neuropathy has mostly been extrapolated from validated studies on diabetic neuropathy. The first line treatment for Chemotherapy Induced Neuropathic Pain includes antidepressants and anticonvulsants, which if not effective on their own, can be combined with opioids. TriCyclic Antidepressants (TCA’s) such as Amitriptyline and Nortriptyline (PAMELOR®) can be considered as first line choice for appropriate patients, although its mechanism of action is uncertain and 20% of the patients discontinue therapy because of adverse effects.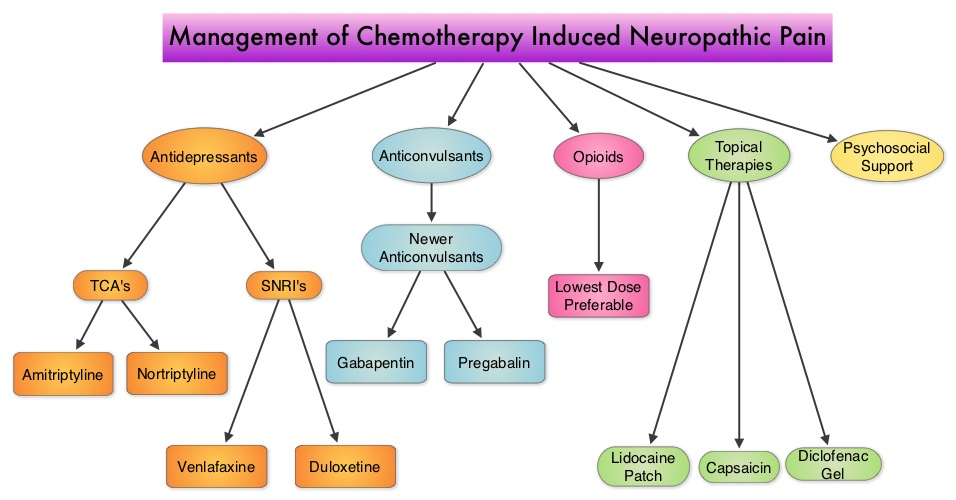 Patients may be able to better tolerate these agents if started at a lower dose and dose titrated slowly every three to five days. Peripheral neuropathic pain has been attributed to an unbalanced release of norepinephrine and serotonin from the neurons. Serotonin-Norepinephrine Reuptake Inhibitors (SNRI’s), including Venlafaxine (EFFEXOR®) and Duloxetine (CYMBALTA®), are better tolerated and have fewer drug interactions than TCA’s. EFFEXOR® in one study significantly relieved Oxaliplatin induced neuropathic pain in more than two thirds of the patients when compared to placebo and a third of the patients had complete pain relief. There is however limited evidence showing a beneficial role of Selective Serotonin Reuptake Inhibitors (SSRI’s) such as Citalopram (CELEXA®) and Paroxetine (PAXIL®) for neuropathic pain. As a note of caution, SNRI’s such as EFFEXOR® and CYMBALTA®, can interact with Tamoxifen, prescribed to patients with breast cancer, preventing Tamoxifen from converting to its active form. The dose of antidepressants needed to alleviate neuropathic pain is not dependent on antidepressant activity and may be lower than that recommended for treatment of depression. The newer anticonvulsants such as Gabapentin (NEURONTIN®), Pregabalin (LYRICA®) are preferable first line agents for the treatment of neuropathic pain rather than traditional, older agents such as Carbamazepine (TEGRETOL®), Phenytoin (DILANTIN®) and Valproate (DEPAKOTE®), as the newer agents are associated with fewer drug interactions. The newer agents bind to the alpha2-delta subunit of the calcium-sensitive channels, modulating neurotransmitter release. Of the newer agents, NEURONTIN® (Gabapentin) is not protein bound and is excreted unchanged in the urine and therefore has fewer drug interactions. If opioids are a consideration for neuropathic pain relief, the lowest dose is recommended. Topical therapies for neuropathic pain have the advantage of controlling pain without systemic side effects. It therefore can be combined with systemic treatment. Lidocaine 5% patches (LIDODERM®) block neuronal sodium channels whereas Capsaicin cream (ZOSTRIX®) stimulates the C fibers to release and subsequently deplete substance P, there by blocking pain signaling to the brain. Diclofenac gel 1% when applied once a day, concentrates in the dermis and has less gastrointestinal side effects and may be beneficial for neuropathic pain. A combination of Ketamine 1% and Amitriptyline 2% cream applied topically has also been promising in a small study. Patients experiencing refractory pain may benefit with the use of Transcutaneous Electrical Nerve Stimulation (TENS), although referral to the pain clinic may be appropriate. Psychosocial support utilizing a team of specialists and social workers/counsellors, should be an integral part of pain management. Kvale E and Urba SG. National Comprehensive Cancer Network (NCCN) 19th Annual Conference, March 13 – 15, 2014; Hollywood, Florida
Patients may be able to better tolerate these agents if started at a lower dose and dose titrated slowly every three to five days. Peripheral neuropathic pain has been attributed to an unbalanced release of norepinephrine and serotonin from the neurons. Serotonin-Norepinephrine Reuptake Inhibitors (SNRI’s), including Venlafaxine (EFFEXOR®) and Duloxetine (CYMBALTA®), are better tolerated and have fewer drug interactions than TCA’s. EFFEXOR® in one study significantly relieved Oxaliplatin induced neuropathic pain in more than two thirds of the patients when compared to placebo and a third of the patients had complete pain relief. There is however limited evidence showing a beneficial role of Selective Serotonin Reuptake Inhibitors (SSRI’s) such as Citalopram (CELEXA®) and Paroxetine (PAXIL®) for neuropathic pain. As a note of caution, SNRI’s such as EFFEXOR® and CYMBALTA®, can interact with Tamoxifen, prescribed to patients with breast cancer, preventing Tamoxifen from converting to its active form. The dose of antidepressants needed to alleviate neuropathic pain is not dependent on antidepressant activity and may be lower than that recommended for treatment of depression. The newer anticonvulsants such as Gabapentin (NEURONTIN®), Pregabalin (LYRICA®) are preferable first line agents for the treatment of neuropathic pain rather than traditional, older agents such as Carbamazepine (TEGRETOL®), Phenytoin (DILANTIN®) and Valproate (DEPAKOTE®), as the newer agents are associated with fewer drug interactions. The newer agents bind to the alpha2-delta subunit of the calcium-sensitive channels, modulating neurotransmitter release. Of the newer agents, NEURONTIN® (Gabapentin) is not protein bound and is excreted unchanged in the urine and therefore has fewer drug interactions. If opioids are a consideration for neuropathic pain relief, the lowest dose is recommended. Topical therapies for neuropathic pain have the advantage of controlling pain without systemic side effects. It therefore can be combined with systemic treatment. Lidocaine 5% patches (LIDODERM®) block neuronal sodium channels whereas Capsaicin cream (ZOSTRIX®) stimulates the C fibers to release and subsequently deplete substance P, there by blocking pain signaling to the brain. Diclofenac gel 1% when applied once a day, concentrates in the dermis and has less gastrointestinal side effects and may be beneficial for neuropathic pain. A combination of Ketamine 1% and Amitriptyline 2% cream applied topically has also been promising in a small study. Patients experiencing refractory pain may benefit with the use of Transcutaneous Electrical Nerve Stimulation (TENS), although referral to the pain clinic may be appropriate. Psychosocial support utilizing a team of specialists and social workers/counsellors, should be an integral part of pain management. Kvale E and Urba SG. National Comprehensive Cancer Network (NCCN) 19th Annual Conference, March 13 – 15, 2014; Hollywood, Florida
A Less Intense Schedule of ZOMETA® for Patients with Metastatic Breast Cancer
Bisphosphonates inhibit osteoclast-mediated bone resorption and both oral and IV bisphosphonates reduce the risk of developing Skeletal Related Events (SRE’s) and delay the time to SRE’s in patients with Breast Cancer with bone metastases. In a study presented at ASCO 2014 meeting, continuing ZOMETA® (Zoledronic acid) for an additional year at the every 12 week schedule was non-inferior to ZOMETA® given every 4 weeks, among patients who had initially received IV bisphosphonates monthly, for one year or longer. This less frequent dosing of ZOMETA® compared with the standard monthly dosing, may be more convenient for the patients and result in less toxicities without compromising efficacy. More information at www.oncoprescribe.com
