SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 24,050 new cases will be diagnosed in 2014 and 11,090 will die of the disease. Maintenance or Continuous Treatment in patients with newly diagnosed multiple myeloma following induction and consolidation can result in significantly longer Progression Free Survival (PFS) and Overall Survival (OS), compared to those patients who receive therapy for a fixed duration of time. Not all studies however, have shown Overall Survival benefit. It has been hypothesized that Continuous Treatment could result in resistance to therapy which in turn could reduce the duration of subsequent remission after first relapse and negatively impact overall survival.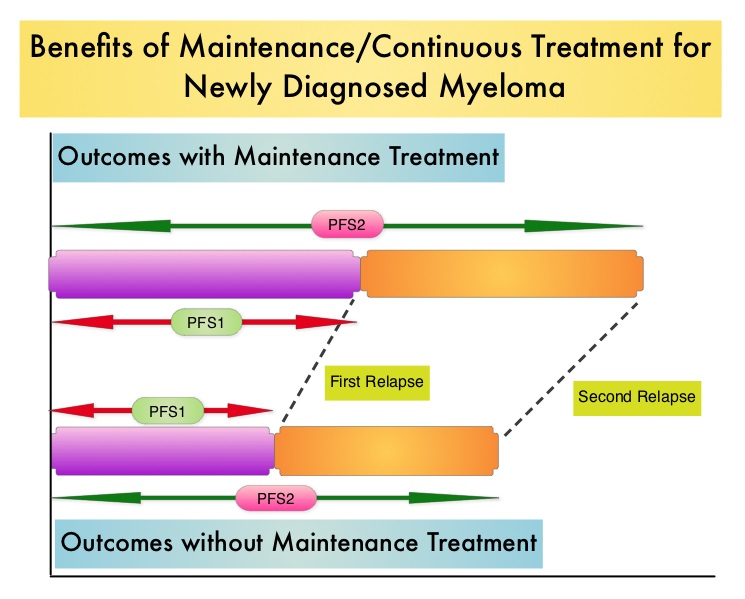 To address this controversy, the authors conducted a pooled analysis of the outcomes of two randomized phase III trials, designed to compare Continuous Treatment to Fixed Duration Therapy. In trail RVMM209, patients were randomized to either induction with Lenolidomide (REVLIMID®), followed by consolidation and subsequent maintenance with REVLIMID® (Continuous Treatment) or Fixed Duration Therapy which entailed REVLIMID® based induction followed by consolidation but no maintenance therapy. In Trial GIMEMA0305, the randomization was between Bortezomib (VELCADE®) based induction followed by maintenance treatment (Continuous Treatment) and VELCADE® induction, with no maintenance treatment (Fixed Duration Therapy). The trial investigators assessed PFS1 as the time from diagnosis to the occurrence of 1st relapse, PFS2 as time from diagnosis to the occurrence of 2nd relapse and Overall Survival as time from diagnosis to death , incorporating the duration of both 1st and 2nd remission. They then evaluated, both PFS1, PFS2 and OS in newly diagnosed multiple myeloma patients who received Continuous Therapy or Fixed Duration Therapy. In this pooled analysis 452 patients received Continuous Treatment and 461 patients received Fixed Duration Therapy .The median follow up was 52 months. Patients receiving Continuous Treatment had significantly prolonged PFS1 (median 35 months vs 24 months, HR 0.58; P<0.0001), PFS2 (median 63 months vs 47 months, HR 0.69, P=0.0001) and OS (median not reached [NR] vs 70 months, HR 0.70, P=0.0019), when compared with Fixed Dose Therapy. The authors evaluated the PFS and OS from first relapse to second relapse and from first relapse to death respectively, and they noted that the outcomes were similar among patients who received Continuous Treatment or Fixed Dose Therapy following initial diagnosis. The authors concluded that Continuous Treatment significantly improved PFS1, PFS2, and OS and findings from this pooled analysis suggested that the clinical benefit observed during first remission was not negated by a shorter second remission and Continuous Treatment did not induce tumor resistance. Continuous Treatment may be essential, as patients with multiple myeloma will always have some residual disease. It should be noted that certain institutions including the Mayo Clinic cap Continuous/Maintenance treatment at approximately 2 years, due to the lack of randomized comparative data, on the value of prolonged maintenance beyond 2 years. Palumbo A, Gay F, Musto P, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8515)
To address this controversy, the authors conducted a pooled analysis of the outcomes of two randomized phase III trials, designed to compare Continuous Treatment to Fixed Duration Therapy. In trail RVMM209, patients were randomized to either induction with Lenolidomide (REVLIMID®), followed by consolidation and subsequent maintenance with REVLIMID® (Continuous Treatment) or Fixed Duration Therapy which entailed REVLIMID® based induction followed by consolidation but no maintenance therapy. In Trial GIMEMA0305, the randomization was between Bortezomib (VELCADE®) based induction followed by maintenance treatment (Continuous Treatment) and VELCADE® induction, with no maintenance treatment (Fixed Duration Therapy). The trial investigators assessed PFS1 as the time from diagnosis to the occurrence of 1st relapse, PFS2 as time from diagnosis to the occurrence of 2nd relapse and Overall Survival as time from diagnosis to death , incorporating the duration of both 1st and 2nd remission. They then evaluated, both PFS1, PFS2 and OS in newly diagnosed multiple myeloma patients who received Continuous Therapy or Fixed Duration Therapy. In this pooled analysis 452 patients received Continuous Treatment and 461 patients received Fixed Duration Therapy .The median follow up was 52 months. Patients receiving Continuous Treatment had significantly prolonged PFS1 (median 35 months vs 24 months, HR 0.58; P<0.0001), PFS2 (median 63 months vs 47 months, HR 0.69, P=0.0001) and OS (median not reached [NR] vs 70 months, HR 0.70, P=0.0019), when compared with Fixed Dose Therapy. The authors evaluated the PFS and OS from first relapse to second relapse and from first relapse to death respectively, and they noted that the outcomes were similar among patients who received Continuous Treatment or Fixed Dose Therapy following initial diagnosis. The authors concluded that Continuous Treatment significantly improved PFS1, PFS2, and OS and findings from this pooled analysis suggested that the clinical benefit observed during first remission was not negated by a shorter second remission and Continuous Treatment did not induce tumor resistance. Continuous Treatment may be essential, as patients with multiple myeloma will always have some residual disease. It should be noted that certain institutions including the Mayo Clinic cap Continuous/Maintenance treatment at approximately 2 years, due to the lack of randomized comparative data, on the value of prolonged maintenance beyond 2 years. Palumbo A, Gay F, Musto P, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8515)
Month: August 2014
Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse
SUMMARY:Prostate cancer is the most common cancer in American men, excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, over 230,000 new cases of prostate cancer will be diagnosed in 2014 and close to 30,000 men will die of the disease. The major source of PSA (Prostate Specific Antigen) is the prostate gland and the PSA levels are therefore undetectable within 6 weeks after Radical Prostatectomy. Similarly, following Radiation Therapy, there is a gradual decline in PSA before reaching a post treatment nadir. A detectable PSA level after Radical Prostatectomy, or a rising PSA level following Radiation Therapy, is considered PSA failure or biochemical recurrence.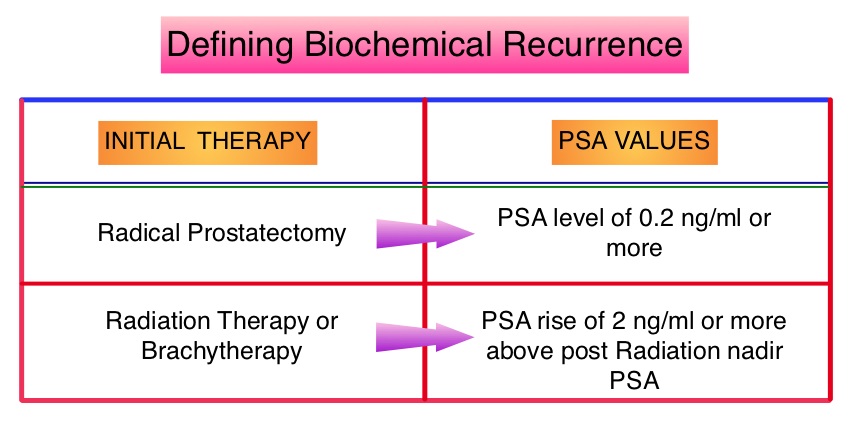 The American Urological Association suggested that a PSA of 0.2 ng/mL or higher defines PSA failure or relapse, after Radical Prostatectomy. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir, is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. Prostate cancer patients who had thought that they were cured, consequently can experience considerable mental anguish and anxiety, based on these laboratory findings. Androgen Deprivation Therapy (ADT) is often initiated following PSA only relapse with the intent of delaying disease progression although the role of ADT and optimal timing to start ADT (Immediate vs deferred ADT) in this patient population is unknown. Further, ADT can be associated with side effects such as fatigue, loss of muscle mass, impotence, anemia, osteoporosis, etc., which in turn can have a significant negative impact on an individual’s quality of life. In order to determine the significance of benefit if any, with starting ADT while patients are asymptomatic, the authors analyzed data on more than 14,000 patients included in a prospective registry called CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) and of them studied 2,022 men, who had experienced a PSA only relapse following curative surgery or radiation. These patients had clinical stage T3a,N0M0 or lower stage prostate cancer and experienced PSA only relapse (defined as PSA of 0.2 ng/mL or higher after Radical Prostatectomy or three rising PSA values one month apart following radiation treatment. Patients with symptoms, documented metastatic disease by CT scan or bone scan and ADT in the previous 12 months were excluded. Patients in the” Immediate treatment group” initiated ADT within 3 months of PSA relapse and those in the “Deferred treatment group” initiated ADT, 2 or more years after PSA relapse or when they presented with metastasis, symptoms or had a short PSA doubling time. The median age was 69 years, 34% had a Gleason score >7 and 32% received radiotherapy as primary treatment. The median time from primary treatment to PSA relapse was 27 months. Patients were followed for a median of 52.3 months after PSA relapse. The Five-year survival rate for Patients in the” Immediate treatment group” was 85.1% and for those in the “Deferred treatment group” was 87.2% with no significant difference in the all cause mortality. The 10 year survival was identical in both groups at 71.6%. The authors concluded that there is little or no survival benefit for Immediate ADT initiation compared with Deferred ADT initiation (at clinical progression or at least two years after PSA relapse) among prostate cancer patients with PSA only relapse. Therefore delaying ADT for at least 2 years after PSA relapse, following curative therapy for prostate cancer does not worsen overall survival. The findings from this large observational study will need further validation and a randomized phase III trial is underway to confirm these findings. Garcia-Albeniz X, Chan JM, Paciorek AT, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 5003)
The American Urological Association suggested that a PSA of 0.2 ng/mL or higher defines PSA failure or relapse, after Radical Prostatectomy. A PSA rise of 2 ng/ml or more above post Radiation Therapy nadir, is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. Prostate cancer patients who had thought that they were cured, consequently can experience considerable mental anguish and anxiety, based on these laboratory findings. Androgen Deprivation Therapy (ADT) is often initiated following PSA only relapse with the intent of delaying disease progression although the role of ADT and optimal timing to start ADT (Immediate vs deferred ADT) in this patient population is unknown. Further, ADT can be associated with side effects such as fatigue, loss of muscle mass, impotence, anemia, osteoporosis, etc., which in turn can have a significant negative impact on an individual’s quality of life. In order to determine the significance of benefit if any, with starting ADT while patients are asymptomatic, the authors analyzed data on more than 14,000 patients included in a prospective registry called CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) and of them studied 2,022 men, who had experienced a PSA only relapse following curative surgery or radiation. These patients had clinical stage T3a,N0M0 or lower stage prostate cancer and experienced PSA only relapse (defined as PSA of 0.2 ng/mL or higher after Radical Prostatectomy or three rising PSA values one month apart following radiation treatment. Patients with symptoms, documented metastatic disease by CT scan or bone scan and ADT in the previous 12 months were excluded. Patients in the” Immediate treatment group” initiated ADT within 3 months of PSA relapse and those in the “Deferred treatment group” initiated ADT, 2 or more years after PSA relapse or when they presented with metastasis, symptoms or had a short PSA doubling time. The median age was 69 years, 34% had a Gleason score >7 and 32% received radiotherapy as primary treatment. The median time from primary treatment to PSA relapse was 27 months. Patients were followed for a median of 52.3 months after PSA relapse. The Five-year survival rate for Patients in the” Immediate treatment group” was 85.1% and for those in the “Deferred treatment group” was 87.2% with no significant difference in the all cause mortality. The 10 year survival was identical in both groups at 71.6%. The authors concluded that there is little or no survival benefit for Immediate ADT initiation compared with Deferred ADT initiation (at clinical progression or at least two years after PSA relapse) among prostate cancer patients with PSA only relapse. Therefore delaying ADT for at least 2 years after PSA relapse, following curative therapy for prostate cancer does not worsen overall survival. The findings from this large observational study will need further validation and a randomized phase III trial is underway to confirm these findings. Garcia-Albeniz X, Chan JM, Paciorek AT, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 5003)
Final results of a randomized phase 2 study showing the clinical benefit of quizartinib (AC220) in patients with FLT3-ITD positive relapsed or refractory acute myeloid leukemia
SUMMARY: Acute Myeloid Leukemia (AML) is generally a disease of the elderly and the average age of a patient at the time of diagnosis is about 66 years. According to the American Cancer Society, approximately 18,860 new cases of AML will be diagnosed in 2014 and 10,460 patients will die of the disease. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy. 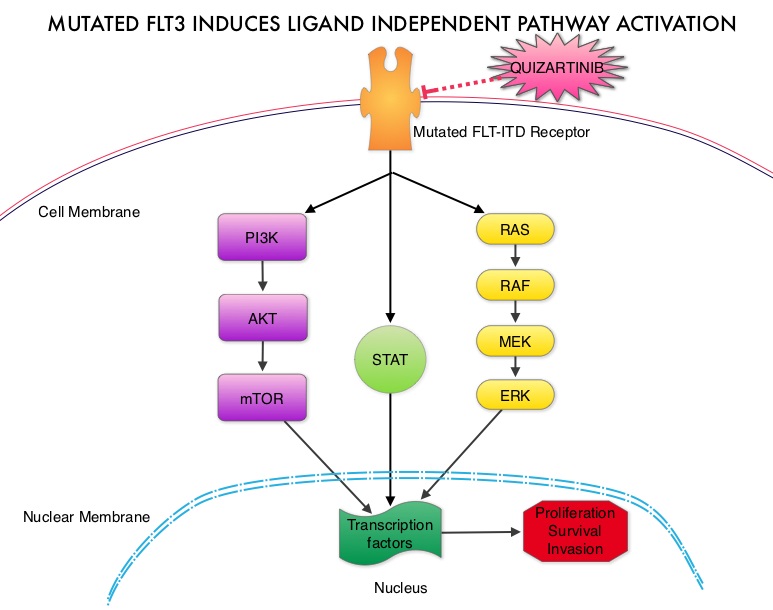 The fms-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by a tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. Several therapeutic agents are being developed to target FLT3 mutations. Quizartinib (AC220) is an oral tyrosine kinase inhibitor, which has demonstrated activity in patients with both wild type FLT3 as well as those with FLT3-ITD mutations. In this randomized, open label phase II study, the authors evaluated the efficacy and safety of two different, lower dosages of Quizartinib, in patients 18 years of age or older, with FLT3-ITD positive, relapsed or refractory AML. Seventy six patients (N=76) were randomized to receive either Quizartinib 30 mg/day (Group A) or Quizartinib 60 mg/day (Group B), given orally and continuously, during a 28 day treatment cycle. Treatment was continued until relapse, intolerance or Hematopoietic Stem Cell Transplantation (HSCT). Both groups were well balanced except for age over 60 years (42% Group A, 26% Group B) and the percentage with secondary AML (8% Group A, 18% Group B). The composite Complete Remission (CRc) rate included Complete Remission (CR), Complete Remission with incomplete platelet recovery (CRp), and Complete Remission with incomplete hematologic recovery (CRi). The CRc rate in both groups A and B was 47% and the Overall Response Rate (CRc + Partial Response (PR)) was 61% in Group A and 71% in Group B. Further, 32% of patiens in Group A and 42% in Group B were able to undergo HSCT, after achieving CRc or PR. The most common treatment related adverse events were diarrhea (18%), febrile neutropenia (16%), and QT prolongation (15%). The QT prolongation rate was significantly less with lower doses of Quizartinib, as was used in this study, compared to what was noted with higher doses of Quizartinib utilized in other trials. The authors concluded that Quizartinib is highly effective in relapsed and refractory AML patients with FLT3-ITD mutations, with an acceptable safety profile. Schiller GJ, Tallman MS, Goldberg SL, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7100)
The fms-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by a tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. Several therapeutic agents are being developed to target FLT3 mutations. Quizartinib (AC220) is an oral tyrosine kinase inhibitor, which has demonstrated activity in patients with both wild type FLT3 as well as those with FLT3-ITD mutations. In this randomized, open label phase II study, the authors evaluated the efficacy and safety of two different, lower dosages of Quizartinib, in patients 18 years of age or older, with FLT3-ITD positive, relapsed or refractory AML. Seventy six patients (N=76) were randomized to receive either Quizartinib 30 mg/day (Group A) or Quizartinib 60 mg/day (Group B), given orally and continuously, during a 28 day treatment cycle. Treatment was continued until relapse, intolerance or Hematopoietic Stem Cell Transplantation (HSCT). Both groups were well balanced except for age over 60 years (42% Group A, 26% Group B) and the percentage with secondary AML (8% Group A, 18% Group B). The composite Complete Remission (CRc) rate included Complete Remission (CR), Complete Remission with incomplete platelet recovery (CRp), and Complete Remission with incomplete hematologic recovery (CRi). The CRc rate in both groups A and B was 47% and the Overall Response Rate (CRc + Partial Response (PR)) was 61% in Group A and 71% in Group B. Further, 32% of patiens in Group A and 42% in Group B were able to undergo HSCT, after achieving CRc or PR. The most common treatment related adverse events were diarrhea (18%), febrile neutropenia (16%), and QT prolongation (15%). The QT prolongation rate was significantly less with lower doses of Quizartinib, as was used in this study, compared to what was noted with higher doses of Quizartinib utilized in other trials. The authors concluded that Quizartinib is highly effective in relapsed and refractory AML patients with FLT3-ITD mutations, with an acceptable safety profile. Schiller GJ, Tallman MS, Goldberg SL, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7100)
Belinostat, a novel pan-histone deacetylase inhibitor (HDACi), in relapsed or refractory peripheral T-cell lymphoma (R/R PTCL) Results from the BELIEF trial
SUMMARY: Non-Hodgkin lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. T cell Lymphomas are a heterogenous group of lymphoid malignancies representing less than 15% of all Non-Hodgkin Lymphomas.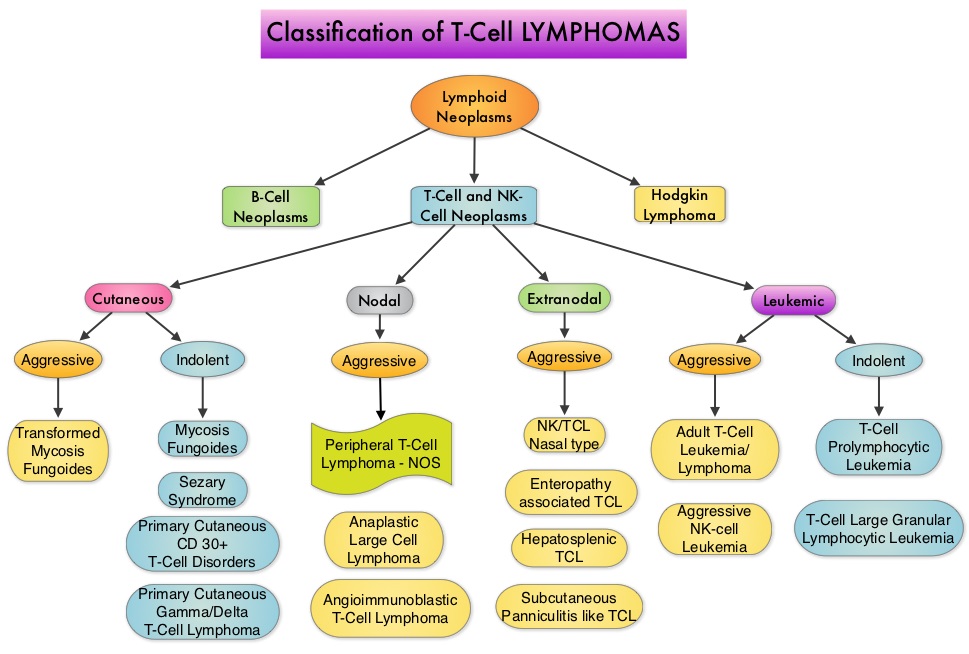
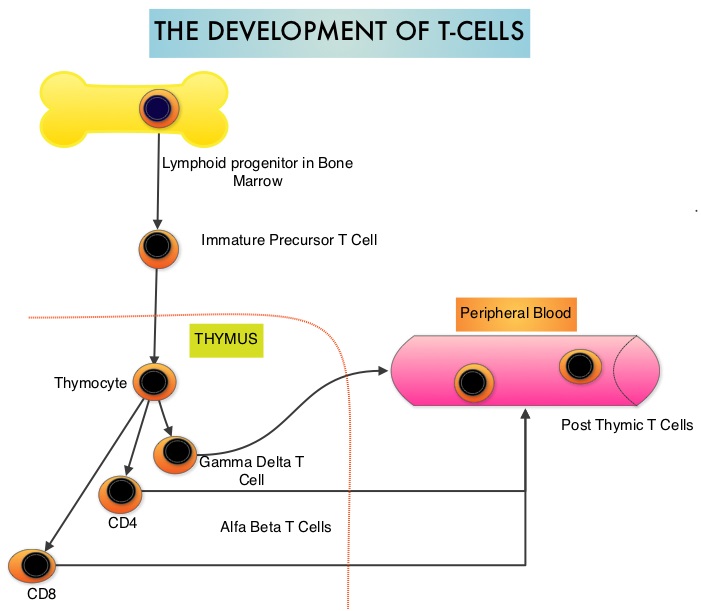 Peripheral T-cell lymphoma – NOS (PTCL-Not Otherwise Specified) is the most common of the aggressive T-cell lymphoma subtypes and accounts for 26% of all non-cutaneous PTCL’s. These malignancies are derived from mature post thymic T-cells and NK cells. These tumors are uncommon, tend to be aggressive and majority of the patients with T-cell lymphoma present with advanced stage disease and respond poorly to treatment. Relapse rates tend to be high and few patients achieve durable remission with treatment. For these reasons, prognosis remains poor. Agents from two pharmacological classes are presently available for the treatment of PTCL. The FDA granted accelerated approval to FOLOTYN® (Pralatrexate), an antifolate, in 2009, for use in patients with relapsed or refractory PTCL and to ISTODAX® (Romidepsin), a histone deacetylase (HDAC) inhibitor in 2011, for the treatment of PTCL patients, who had received at least one prior therapy.
Peripheral T-cell lymphoma – NOS (PTCL-Not Otherwise Specified) is the most common of the aggressive T-cell lymphoma subtypes and accounts for 26% of all non-cutaneous PTCL’s. These malignancies are derived from mature post thymic T-cells and NK cells. These tumors are uncommon, tend to be aggressive and majority of the patients with T-cell lymphoma present with advanced stage disease and respond poorly to treatment. Relapse rates tend to be high and few patients achieve durable remission with treatment. For these reasons, prognosis remains poor. Agents from two pharmacological classes are presently available for the treatment of PTCL. The FDA granted accelerated approval to FOLOTYN® (Pralatrexate), an antifolate, in 2009, for use in patients with relapsed or refractory PTCL and to ISTODAX® (Romidepsin), a histone deacetylase (HDAC) inhibitor in 2011, for the treatment of PTCL patients, who had received at least one prior therapy.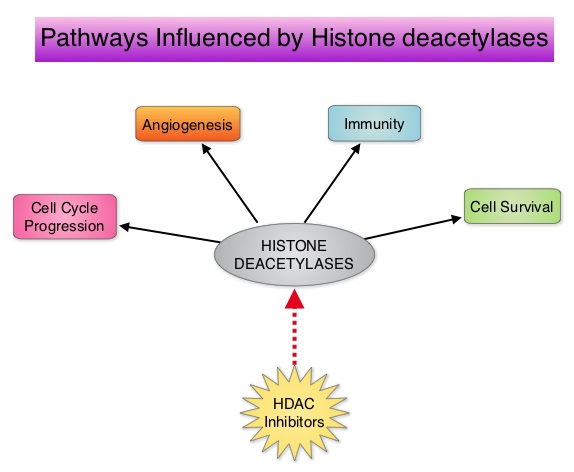 HDACs are a family of enzymes that play an important role in the regulation of gene expression. To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes and regulate cell-cycle progression, cell survival, angiogenesis and immunity. BELEODAQ® (Belinostat) is a novel pan-histone deacetylase inhibitor and inhibits all 3 classes of the zinc-dependent HDAC enzymes.
HDACs are a family of enzymes that play an important role in the regulation of gene expression. To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes and regulate cell-cycle progression, cell survival, angiogenesis and immunity. BELEODAQ® (Belinostat) is a novel pan-histone deacetylase inhibitor and inhibits all 3 classes of the zinc-dependent HDAC enzymes.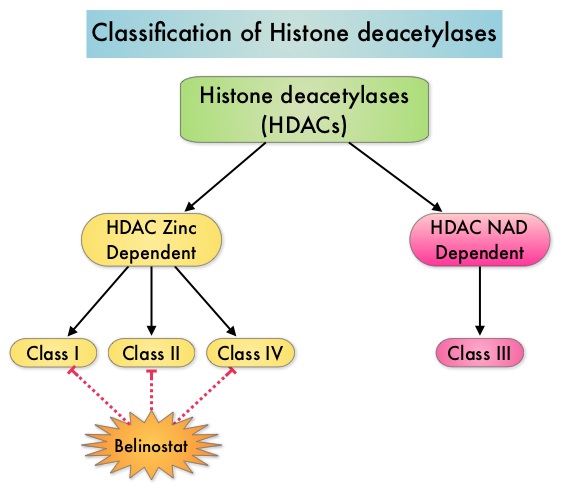 The approval of BELEODAQ® was based on the results of a multi-center, single-arm, phase II trial, in which 129 patients with relapsed or refractory PTCL, who had received a median of 2 prior therapies, were enrolled and evaluated. BELEODAQ® was administered as a 30 minute IV infusion at 1000 mg/m2 on days 1–5 of a 3 week cycle until progression or unacceptable toxicity. The median age was 63 years. The primary endpoint was Overall Response Rate (ORR). The ORR was 26% with 10% Complete Responses and 10% Partial responses and the median time to response was 5.6 weeks and the median duration of response was 8.3 months. The most common adverse events were nausea, vomiting, fatigue, fever and anemia. The most common grade 3/4 adverse events were thrombocytopenia (13%), neutropenia (13%), anemia (10%), dyspnea (6%), pneumonia (6%), and fatigue (5%). The authors concluded that BELEODAQ® demonstrated a significant overall response rate in relapsed /refractory PTCL patients, thus expanding the treatment options for these difficult to treat individuals. This was accomplished with a low incidence of myelosuppression and favorable safety profile. O'Connor OA, Masszi T, Savage KJ, et al. J Clin Oncol 31, 2013 (suppl; abstr 8507)
The approval of BELEODAQ® was based on the results of a multi-center, single-arm, phase II trial, in which 129 patients with relapsed or refractory PTCL, who had received a median of 2 prior therapies, were enrolled and evaluated. BELEODAQ® was administered as a 30 minute IV infusion at 1000 mg/m2 on days 1–5 of a 3 week cycle until progression or unacceptable toxicity. The median age was 63 years. The primary endpoint was Overall Response Rate (ORR). The ORR was 26% with 10% Complete Responses and 10% Partial responses and the median time to response was 5.6 weeks and the median duration of response was 8.3 months. The most common adverse events were nausea, vomiting, fatigue, fever and anemia. The most common grade 3/4 adverse events were thrombocytopenia (13%), neutropenia (13%), anemia (10%), dyspnea (6%), pneumonia (6%), and fatigue (5%). The authors concluded that BELEODAQ® demonstrated a significant overall response rate in relapsed /refractory PTCL patients, thus expanding the treatment options for these difficult to treat individuals. This was accomplished with a low incidence of myelosuppression and favorable safety profile. O'Connor OA, Masszi T, Savage KJ, et al. J Clin Oncol 31, 2013 (suppl; abstr 8507)
