SUMMARY: Approximately 32 million Americans take a statin in the United States. Statins (3-Hydroxy-3-MethylGlutaryl coenzyme A reductase inhibitors) are usually prescribed to lower LDL cholesterol. Cholesterol is a structural component of cell membranes and a reduction in the availability of cholesterol can result in decreased proliferation and migration of cancer cells. The six statin drugs available in the United States include LIPITOR® (Atorvastatin), ZOCOR® (Simvastatin), CRESTOR® (Rosuvastatin), MEVACOR® (Lovastatin), PRAVACHOL® (Pravastatin) and LESCOL® (Fluvastatin). 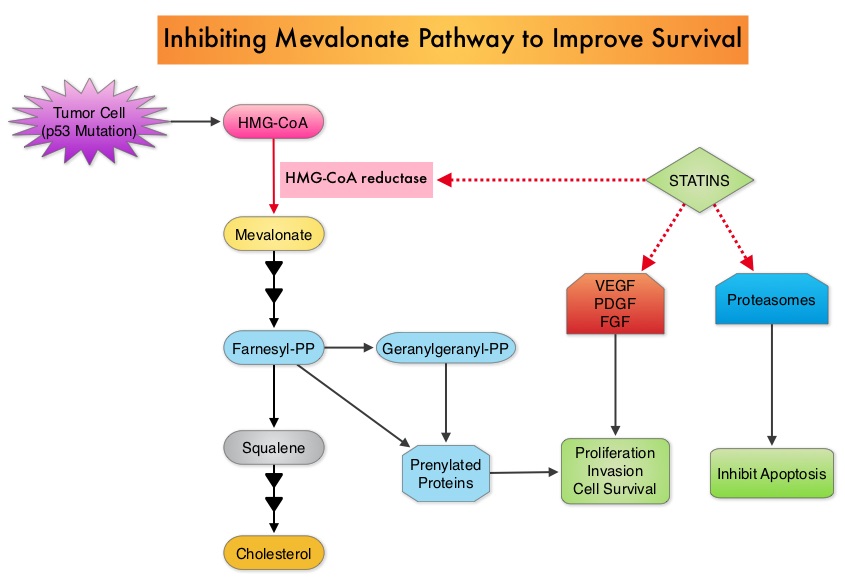 Statin use in cancer patients has been associated with a reduction in cancer related mortality in several clinical studies. This benefit has been attributed to the inhibition of HMG-CoA reductase, which is a rate limiting enzyme in the mevalonate and cholesterol synthesis pathway. The mevalonate pathway is upregulated by mutated p53 (tumor suppressor gene) which is often expressed in cancer cells. By inhibiting the mevalonate pathway, statins can reduce isoprenoid levels such as farnesylpyrophosphate (F-PP) and geranylgeranylpyrophosphate (GG-PP). These isoprenoids are essential for the posttranslational modification of several proteins involved in important intracellular signaling pathways and therefore play a crucial role in cell growth, proliferation, survival and migration. Statins also inhibit angiogenic pathways and proteasomes, thereby negatively impacting cell proliferation and survival. Survival benefit with statin use after colorectal cancer diagnosis has been unclear. To answer this question, the authors identified a cohort of patients (N=7657) diagnosed with stage I to III colorectal cancer from 1998 to 2009, in the National Cancer Data Repository (English Cancer Registry). Information on statin use was obtained from medical records of patients and in this cohort of patients 35% were identified to have used statin drugs following diagnosis of colorectal cancer. Twenty percent of these patients had stage I disease, 43% had stage II disease and 37% had stage III disease. Patients were followed up for 14 years following their diagnosis of colorectal cancer. Statin use after a diagnosis of colorectal cancer was associated with a 29% reduction in colorectal cancer-specific mortality (HR= 0.71). There was a dose-response association with a 36% reduction in colorectal cancer-specific mortality with statin use for more than 1 year (HR=0.64). Statin users after colorectal cancer diagnosis also had a 25% reduction in all-cause mortality (HR=0.75). The authors concluded that based on this large population based colorectal cancer cohort, statin use following diagnosis of colorectal cancer was associated with longer rates of survival. Cardwell CR, Hicks BM, Hughes C, et al. J Clin Oncol 2014;32:3177-3183
Statin use in cancer patients has been associated with a reduction in cancer related mortality in several clinical studies. This benefit has been attributed to the inhibition of HMG-CoA reductase, which is a rate limiting enzyme in the mevalonate and cholesterol synthesis pathway. The mevalonate pathway is upregulated by mutated p53 (tumor suppressor gene) which is often expressed in cancer cells. By inhibiting the mevalonate pathway, statins can reduce isoprenoid levels such as farnesylpyrophosphate (F-PP) and geranylgeranylpyrophosphate (GG-PP). These isoprenoids are essential for the posttranslational modification of several proteins involved in important intracellular signaling pathways and therefore play a crucial role in cell growth, proliferation, survival and migration. Statins also inhibit angiogenic pathways and proteasomes, thereby negatively impacting cell proliferation and survival. Survival benefit with statin use after colorectal cancer diagnosis has been unclear. To answer this question, the authors identified a cohort of patients (N=7657) diagnosed with stage I to III colorectal cancer from 1998 to 2009, in the National Cancer Data Repository (English Cancer Registry). Information on statin use was obtained from medical records of patients and in this cohort of patients 35% were identified to have used statin drugs following diagnosis of colorectal cancer. Twenty percent of these patients had stage I disease, 43% had stage II disease and 37% had stage III disease. Patients were followed up for 14 years following their diagnosis of colorectal cancer. Statin use after a diagnosis of colorectal cancer was associated with a 29% reduction in colorectal cancer-specific mortality (HR= 0.71). There was a dose-response association with a 36% reduction in colorectal cancer-specific mortality with statin use for more than 1 year (HR=0.64). Statin users after colorectal cancer diagnosis also had a 25% reduction in all-cause mortality (HR=0.75). The authors concluded that based on this large population based colorectal cancer cohort, statin use following diagnosis of colorectal cancer was associated with longer rates of survival. Cardwell CR, Hicks BM, Hughes C, et al. J Clin Oncol 2014;32:3177-3183
Month: October 2014
Cancer of Unknown Primary Site
SUMMARY:Carcinoma of Unknown Primary Site (CUPS) is a heterogeneous clinical pathologic syndrome for which the anatomical site of origin of the primary tumor is clinically undetectable. CUPS accounts for approximately 2% of all advanced malignances annually. The American Cancer Society estimates that about 31,430 cases of Cancer of Unknown Primary site will be diagnosed in 2014 in the United States. The pathobiology of tumors from unknown primary sites is similar to those with detectable primary tumors and therefore may respond to therapies similar to those with easily detectable primary tumors. Historically, the treatment approach for patients with CUPS included broad spectrum empiric chemotherapy. Histological evaluation of the biopsy tissue alone has been the standard practice for decades. With the availability of gene expression profiling assays and advances in ImmunoHistoChemistry staining as well as imaging technology, predicting the tissue of origin of the primary tumor and tailoring therapy accordingly, has improved overall survival in this patient population. Evaluation of a patient with CUPS starts with gathering and incorporating medical information which includes the patient’s gender, medical history, clinical findings and sites of metastases. A CT scan of the chest, abdomen and pelvis with IV and oral contrast is recommended, although PET (Positron Emission Tomography) or an MRI can be performed in those with renal insufficiency or iodine allergy. PET scan is recommended for those with cervical lymphadenopathy with squamous histology, to help determine the extent of the disease and treatment planning for radiation. PET imaging is also helpful for patients with solitary metastases before locoregional therapies are planned, as well as assessing response in patients with predominantly bone only disease. In women presenting with isolated axillary lymphadenopathy, adenocarcinoma histology, negative mammograms and ultrasound, MRI of the breasts is indicated. With the exception of those patients with CUPS who present with cervical lymphadenopathy, diagnostic procedures such as bronchoscopy, EGD and colonoscopy are not recommended in asymptomatic patients. Tumor markers in general do not have diagnostic value in patients with CUPS although they could be utilized to monitor response to treatment. However, PSA when elevated in a male with adenocarcinoma and osteoblastic metastases, is suggestive of a prostate primary. Similarly an elevated Beta HCG and AFP in a patient with undifferentiated or poorly differentiated carcinoma, is suggestive of an extragonadal germ cell tumor and an elevated AFP is also helpful in making a diagnosis of Hepatoma. Approximately 60% of the patients with CUPS have well or moderately differentiated adenocarcinoma on light microscopy, 30% have poorly differentiated carcinoma or adenocarcinoma, 5% have poorly differentiated or undifferentiated malignancy and 5% have squamous cell carcinoma. Following histological evaluation on light microscopy, the biopsy specimen is further tested using ImmunoHistoChemical stains, using peroxidase labeled antibodies against tumor specific antigens, taking advantage of the similarities in the tumor profiles of primary and metastatic malignancies. After delineating a tumor as carcinoma, lymphoma, sarcoma or melanoma, additional IHC testing can help identify tumors such as a lung primary (postive Thyroid Transcription Factor 1-TTF1and positive CytoKeratin 7- CK7), lower gastrointestinal cancers (positive CK20, positive CDX2 and negative CK7) or a breast primary (positive CK7 and positive Mammaglobin). Tissue-of-Origin molecular profiling is based on the principle that in patients with CUPS, molecular signatures of metastatic tumors are similar to their primary tumor. Tissue-of-Origin molecular profiling is performed using tools such as DNA microarray, quantitative real time polymerase chain reaction assay (rt-PCR) or assays based on messenger RNA (mRNA) or microRNA. These tests are cost-effective and 70% – 90% accurate. This study can be performed on formalin-fixed samples as well as samples from fine needle aspiration. Even though platinum based chemotherapy has been the default regimen for patients with CUPS, histological evaluation of biopsy tissue by light microscopy, IHC testing and molecular profiling assay may complement each other and help guide the Health Care Provider to select site specific therapy. The survival outcomes of CUPS patients with a Tissue-of-Origin molecularly diagnosed profile are comparable to those with similar type advanced cancer with a known primary. The authors concluded that with additional molecular insights into tumor biology and availability of newer therapeutic agents, patients with CUPS and known primary tumors may eventually be treated alike. Varadhachary, GR and Raber, MN. N Engl J Med 2014; 371:757-765
Evaluation of a patient with CUPS starts with gathering and incorporating medical information which includes the patient’s gender, medical history, clinical findings and sites of metastases. A CT scan of the chest, abdomen and pelvis with IV and oral contrast is recommended, although PET (Positron Emission Tomography) or an MRI can be performed in those with renal insufficiency or iodine allergy. PET scan is recommended for those with cervical lymphadenopathy with squamous histology, to help determine the extent of the disease and treatment planning for radiation. PET imaging is also helpful for patients with solitary metastases before locoregional therapies are planned, as well as assessing response in patients with predominantly bone only disease. In women presenting with isolated axillary lymphadenopathy, adenocarcinoma histology, negative mammograms and ultrasound, MRI of the breasts is indicated. With the exception of those patients with CUPS who present with cervical lymphadenopathy, diagnostic procedures such as bronchoscopy, EGD and colonoscopy are not recommended in asymptomatic patients. Tumor markers in general do not have diagnostic value in patients with CUPS although they could be utilized to monitor response to treatment. However, PSA when elevated in a male with adenocarcinoma and osteoblastic metastases, is suggestive of a prostate primary. Similarly an elevated Beta HCG and AFP in a patient with undifferentiated or poorly differentiated carcinoma, is suggestive of an extragonadal germ cell tumor and an elevated AFP is also helpful in making a diagnosis of Hepatoma. Approximately 60% of the patients with CUPS have well or moderately differentiated adenocarcinoma on light microscopy, 30% have poorly differentiated carcinoma or adenocarcinoma, 5% have poorly differentiated or undifferentiated malignancy and 5% have squamous cell carcinoma. Following histological evaluation on light microscopy, the biopsy specimen is further tested using ImmunoHistoChemical stains, using peroxidase labeled antibodies against tumor specific antigens, taking advantage of the similarities in the tumor profiles of primary and metastatic malignancies. After delineating a tumor as carcinoma, lymphoma, sarcoma or melanoma, additional IHC testing can help identify tumors such as a lung primary (postive Thyroid Transcription Factor 1-TTF1and positive CytoKeratin 7- CK7), lower gastrointestinal cancers (positive CK20, positive CDX2 and negative CK7) or a breast primary (positive CK7 and positive Mammaglobin). Tissue-of-Origin molecular profiling is based on the principle that in patients with CUPS, molecular signatures of metastatic tumors are similar to their primary tumor. Tissue-of-Origin molecular profiling is performed using tools such as DNA microarray, quantitative real time polymerase chain reaction assay (rt-PCR) or assays based on messenger RNA (mRNA) or microRNA. These tests are cost-effective and 70% – 90% accurate. This study can be performed on formalin-fixed samples as well as samples from fine needle aspiration. Even though platinum based chemotherapy has been the default regimen for patients with CUPS, histological evaluation of biopsy tissue by light microscopy, IHC testing and molecular profiling assay may complement each other and help guide the Health Care Provider to select site specific therapy. The survival outcomes of CUPS patients with a Tissue-of-Origin molecularly diagnosed profile are comparable to those with similar type advanced cancer with a known primary. The authors concluded that with additional molecular insights into tumor biology and availability of newer therapeutic agents, patients with CUPS and known primary tumors may eventually be treated alike. Varadhachary, GR and Raber, MN. N Engl J Med 2014; 371:757-765
Clinical and Safety Outcomes Associated With Treatment of Acute Venous Thromboembolism A Systematic Review and Meta-analysis
SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000 – 100,000 deaths. VTE is the third leading cause of cardiovascular mortality with a mortality rate of up to 25% in those with untreated acute pulmonary embolism. For decades, Unfractionated Heparin (UFH) also known as standard heparin along with Vitamin K antagonist (Warfarin) has been the established standard, for the treatment of Acute Venous ThromboEmbolism. 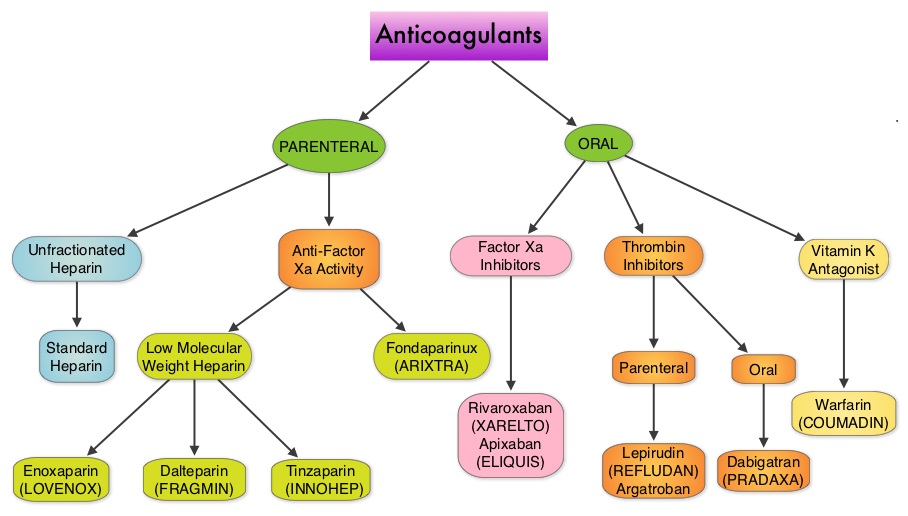 Even though Low Molecular Weight Heparin (LMWH) preparations as well as new oral anticoagulants have been available for the treatment Venous ThromboEmbolism, there has been very little guidance for Health Care Providers on the use of these newer agents. The authors in this analysis compared the efficacy and safety outcomes associated with different anticoagulation regimens for treatment of Venous ThromboEmbolism (VTE). These anticoagulant regimens included Unfractionated Heparin (UFH), Low Molecular Weight Heparin (LMWH) or Fondaparinux in combination with Vitamin K antagonists, LMWH with Dabigatran (PRADAXA®), Rivaroxaban (XARELTO®), Apixaban (ELIQUIS®) or Edoxaban and LMWH alone. This meta analysis included 44,989 patients from 45 randomized trials which reported rates of recurrent VTE and major bleeding in patients with acute VTE. In these Acute Deep Vein Thrombosis and Pulmonary Embolism trials, Rivaroxaban and Apixaban were evaluated without the use of initial LMWH whereas both Dabigatran or Edoxaban were assessed following an initial 5 day treatment with LMWH. This analysis was therefore able to assess clinical and safety outcomes associated with different anticoagulation regimens. The followings findings were noted:
Even though Low Molecular Weight Heparin (LMWH) preparations as well as new oral anticoagulants have been available for the treatment Venous ThromboEmbolism, there has been very little guidance for Health Care Providers on the use of these newer agents. The authors in this analysis compared the efficacy and safety outcomes associated with different anticoagulation regimens for treatment of Venous ThromboEmbolism (VTE). These anticoagulant regimens included Unfractionated Heparin (UFH), Low Molecular Weight Heparin (LMWH) or Fondaparinux in combination with Vitamin K antagonists, LMWH with Dabigatran (PRADAXA®), Rivaroxaban (XARELTO®), Apixaban (ELIQUIS®) or Edoxaban and LMWH alone. This meta analysis included 44,989 patients from 45 randomized trials which reported rates of recurrent VTE and major bleeding in patients with acute VTE. In these Acute Deep Vein Thrombosis and Pulmonary Embolism trials, Rivaroxaban and Apixaban were evaluated without the use of initial LMWH whereas both Dabigatran or Edoxaban were assessed following an initial 5 day treatment with LMWH. This analysis was therefore able to assess clinical and safety outcomes associated with different anticoagulation regimens. The followings findings were noted:
1) Standard Heparin–Vitamin K antagonist combination was associated with an increased risk of recurrent VTE compared with the LMWH–Vitamin K antagonist combination
2) Both new oral anticoagulants and LMWH–vitamin K antagonist combination had similar clinical outcomes. However, the newer oral anticoagulants were associated with a lower risk of major bleeding and this benefit was more pronounced with Rivaroxaban and Apixaban. Compared with LMWH-Dabigatran and LMWH-Edoxaban combinations, Apixaban was associated with a lower risk of bleeding.
This comprehensive analysis lead the authors to conclude that Unfractionated Heparin (Standard Heparin)–Vitamin K antagonist combination is the least effective strategy for the treatment of Acute Venous ThromboEmbolism and Rivaroxaban and Apixaban are associated with the lowest risk for bleeding. Castellucci LA, Cameron C, Le Gal G, et al. JAMA 2014;312:1122-1135
Phase 3 study of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting during repeated moderately emetogenic chemotherapy (MEC) cycles
SUMMARY: Chemotherapy Induced Nausea and Vomiting (CINV) is one of the most common adverse effects of chemotherapy and is experienced by about 80% of patients receiving chemotherapy. The development of effective antiemetic agents has facilitated the administration of majority of the chemotherapy agents in an outpatient setting avoiding hospitalization.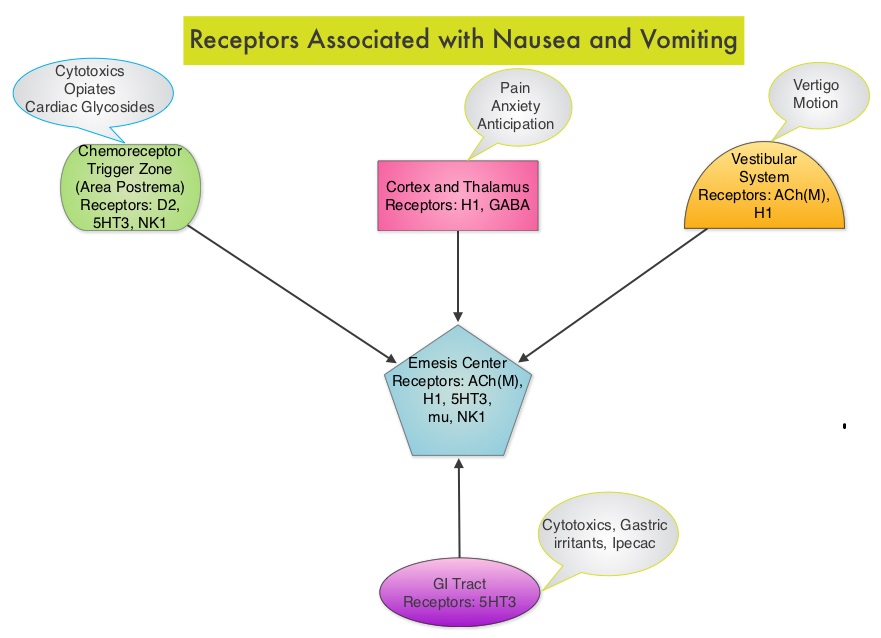 Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.
Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.  Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
Phase 3 study of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting during repeated moderately emetogenic chemotherapy (MEC) cycles
SUMMARY: Chemotherapy Induced Nausea and Vomiting (CINV) is one of the most common adverse effects of chemotherapy and is experienced by about 80% of patients receiving chemotherapy. The development of effective antiemetic agents has facilitated the administration of majority of the chemotherapy agents in an outpatient setting avoiding hospitalization. Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist. Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer
SUMMARY: Prostate cancer is the most common cancer in American men excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, over 230,000 new cases of prostate cancer will be diagnosed in 2014 and close to 30,000 men will die of the disease. Prostate cancer is driven by Androgen Receptor (AR) and its signaling pathways. Initial treatment strategies for patients with metastatic prostate cancer include lowering the levels of circulating androgens with medical or surgical castration or blocking the binding of androgens to the androgen receptor. 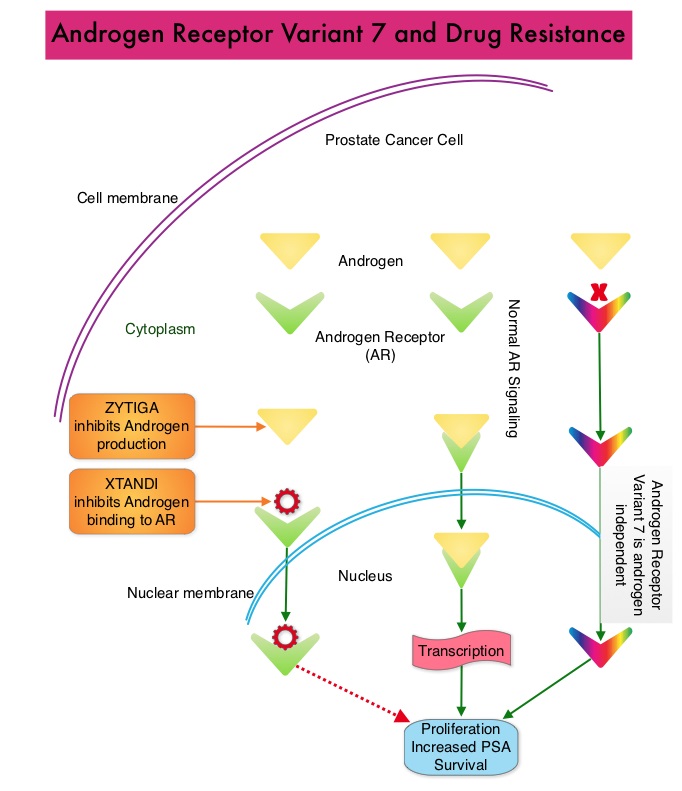 Upon progression {described as Castrate Resistant Prostate Cancer (CRPC), as these tumors are not androgen independent and continue to rely on Androgen Receptor signaling} two agents are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the circulating tumor cells. AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In this prospective study which enrolled patients with Castrate Resistant Prostate Cancer (CRPC), 31 patients received treatment with ZYTIGA® and 31 patients received treatment with XTANDI®. Levels of AR-V7 in circulating tumor cells of these patients were analyzed using quantitative Reverse Transcriptase – Polymerase Chain Reaction assay. The primary endpoint was association between AR-V7 status (positive versus negative) and Prostate Specific Antigen (PSA) response rates and secondary endpoints included freedom from PSA progression (PSA Progression Free Survival), clinical or radiographic Progression Free Survival, and Overall Survival. The authors noted that patients with detectable AR-V7 in circulating tumor cells had no response to ZYTIGA® or XTANDI® as measured by serum PSA level reduction of 50% or more and also had a shorter Progression Free Survival and Overall Survival. Also of interest, the prevalence of detectable AR-V7 in circulating tumor cells before treatment with ZYTIGA® and XTANDI® was 9-15% whereas it increased to approximately 50% after disease progressed during treatment with either of these two drugs. This suggested a common mechanism of resistance to both drugs. The authors concluded that detection of AR-V7 in circulating tumor cells from patients with Castration Resistant Prostate Cancer, may be associated with resistance to ZYTIGA® and XTANDI® and if further validated, could be used as a biomarker. Antonarakis ES, Lu C, Wang H, et al. N Engl J Med 2014; 371:1028-1038
Upon progression {described as Castrate Resistant Prostate Cancer (CRPC), as these tumors are not androgen independent and continue to rely on Androgen Receptor signaling} two agents are presently available for metastatic CRPC. They include ZYTIGA® (Abiraterone) and XTANDI® (Enzalutamide). Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the circulating tumor cells. AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In this prospective study which enrolled patients with Castrate Resistant Prostate Cancer (CRPC), 31 patients received treatment with ZYTIGA® and 31 patients received treatment with XTANDI®. Levels of AR-V7 in circulating tumor cells of these patients were analyzed using quantitative Reverse Transcriptase – Polymerase Chain Reaction assay. The primary endpoint was association between AR-V7 status (positive versus negative) and Prostate Specific Antigen (PSA) response rates and secondary endpoints included freedom from PSA progression (PSA Progression Free Survival), clinical or radiographic Progression Free Survival, and Overall Survival. The authors noted that patients with detectable AR-V7 in circulating tumor cells had no response to ZYTIGA® or XTANDI® as measured by serum PSA level reduction of 50% or more and also had a shorter Progression Free Survival and Overall Survival. Also of interest, the prevalence of detectable AR-V7 in circulating tumor cells before treatment with ZYTIGA® and XTANDI® was 9-15% whereas it increased to approximately 50% after disease progressed during treatment with either of these two drugs. This suggested a common mechanism of resistance to both drugs. The authors concluded that detection of AR-V7 in circulating tumor cells from patients with Castration Resistant Prostate Cancer, may be associated with resistance to ZYTIGA® and XTANDI® and if further validated, could be used as a biomarker. Antonarakis ES, Lu C, Wang H, et al. N Engl J Med 2014; 371:1028-1038
Rituximab Extended Schedule or Re-Treatment Trial for Low–Tumor Burden Follicular Lymphoma Eastern Cooperative Oncology Group Protocol E4402
SUMMARY: Non-Hodgkin Lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. RITUXAN® (Rituximab) is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that destroys malignant human B cells primarily by complement-dependent cytotoxicity (CDC) and Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC).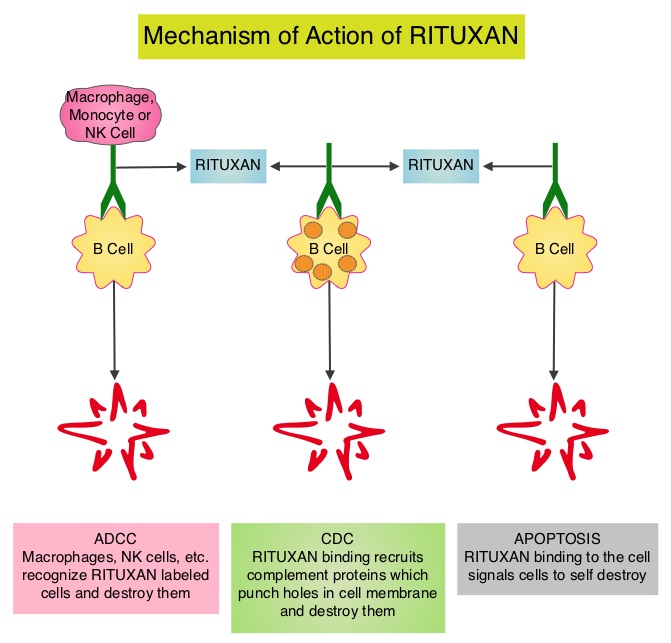 Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102
Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102
Everolimus (EVE) for the treatment of advanced pancreatic neuroendocrine tumors (pNET) Final overall survival (OS) results of a randomized, double-blind, placebo (PBO)-controlled, multicenter Phase III trial (RADIANT-3) SUMMARY Pancreatic NeuroEndocrine Tumors (PNETs) are uncommon, slow growing neoplasms and their incidence has been on the rise due to heightened awareness of the disease, better diagnostic techniques and increased rate of incidental diagnosis during workup for other conditions They account for approximately 25% of all neuroendocrine tumors Pancreatic NETs may be functional or nonfunctional Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer Predictors of worst survival include advanced stage, higher grade and age Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR® Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR® Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR® The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 46 months in the placebo group (P < 0001) The authors now report the mature Overall Survival results The median Overall Survival was 44 months in the AFINITOR® group compared with 377 months in the placebo arm The 63 month survival difference in favor of AFINITOR® was not statistically significant (P= 030) The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR® The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors Yao J, Pavel M, Lombard-Bohas C, et al ESMO 2014 Congress, Abstract#11320
They account for approximately 25% of all neuroendocrine tumors Pancreatic NETs may be functional or nonfunctional Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer Predictors of worst survival include advanced stage, higher grade and age Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR® Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR® Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR® The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 46 months in the placebo group (P < 0001) The authors now report the mature Overall Survival results The median Overall Survival was 44 months in the AFINITOR® group compared with 377 months in the placebo arm The 63 month survival difference in favor of AFINITOR® was not statistically significant (P= 030) The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR® The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors Yao J, Pavel M, Lombard-Bohas C, et al ESMO 2014 Congress, Abstract#11320
SUMMARY: Pancreatic NeuroEndocrine Tumors (PNETs) are uncommon, slow growing neoplasms and their incidence has been on the rise due to heightened awareness of the disease, better diagnostic techniques and increased rate of incidental diagnosis during workup for other conditions.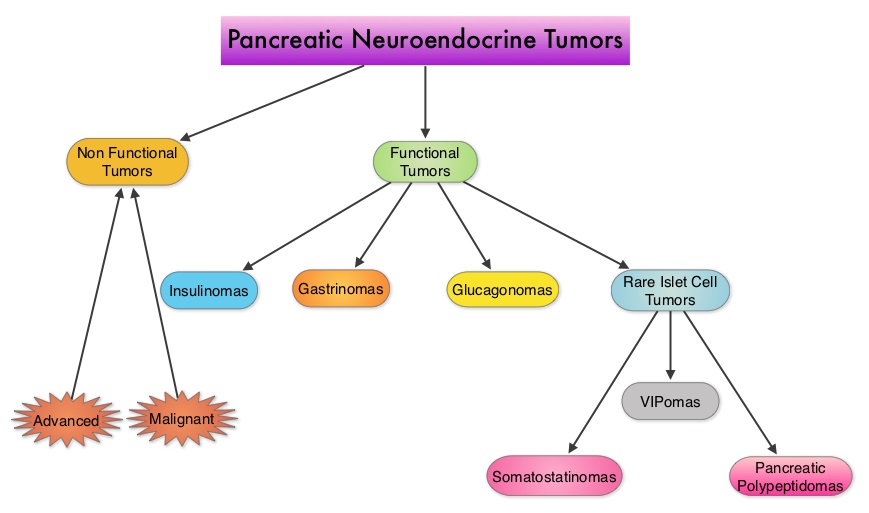 They account for approximately 25% of all neuroendocrine tumors. Pancreatic NETs may be functional or nonfunctional. Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones. Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms. Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer. Predictors of worst survival include advanced stage, higher grade and age. Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor. RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care. The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR®. Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR®. Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR®. The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 4.6 months in the placebo group (P < 0.001). The authors now report the mature Overall Survival results. The median Overall Survival was 44 months in the AFINITOR® group compared with 37.7 months in the placebo arm. The 6.3 month survival difference in favor of AFINITOR® was not statistically significant (P= 0.30). The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR®. The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue. The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors. Yao J, Pavel M, Lombard-Bohas C, et al. ESMO 2014 Congress, Abstract#11320
They account for approximately 25% of all neuroendocrine tumors. Pancreatic NETs may be functional or nonfunctional. Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones. Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms. Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer. Predictors of worst survival include advanced stage, higher grade and age. Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor. RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care. The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR®. Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR®. Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR®. The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 4.6 months in the placebo group (P < 0.001). The authors now report the mature Overall Survival results. The median Overall Survival was 44 months in the AFINITOR® group compared with 37.7 months in the placebo arm. The 6.3 month survival difference in favor of AFINITOR® was not statistically significant (P= 0.30). The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR®. The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue. The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors. Yao J, Pavel M, Lombard-Bohas C, et al. ESMO 2014 Congress, Abstract#11320
Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors vs Conventional Chemotherapy in Non–Small Cell Lung Cancer Harboring Wild-Type Epidermal Growth Factor Receptor – A Meta-analysis
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 224,000 new cases of lung cancer will be diagnosed in the United States in 2014 and over 159,000 will die of the disease. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, adenocarcinoma now is the most frequent histologic subtype of lung cancer.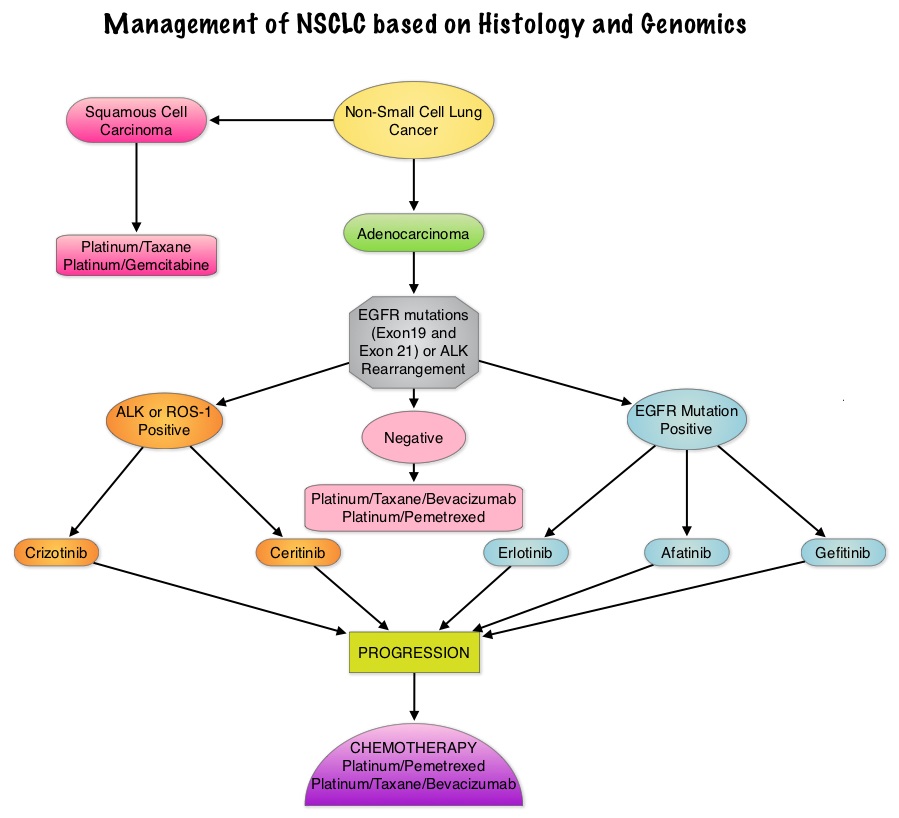 In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced Non Small Cell Lung Cancer (NSCLC) cases with adenocarcinoma histology and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib) and IRESSA® (Gefitinib), changed the treatment paradigm in favor of targeted therapy, for this patient subset. It is estimated that approximately 10% of Western patient population and 50% of Asian patients with NSCLC, harbor EGFR activating mutations. EGFR Tyrosine Kinase Inhibitors have been shown to be superior to conventional chemotherapy in this patient group with improved Progression Free Survival (PFS) and Objective Response Rates. Patients with NSCLC should therefore be tested for the most common sensitizing mutations such as deletions in exon 19 and L858R point mutations in exon 21, as these patients clearly benefit from first line EGFR TKIs. EGFR expression by IHC (ImmunoHistoChemical) staining, EGFR gene copy by FISH (Fluorescence In Situ Hybridization) and blood based proteonomic testing by VERISTRAT® is currently not recommended for the selection of first line EGFR TKIs. There is presently no evidence indicating superiority of TKIs when compared with conventional chemotherapy for the second or third line treatment of EGFR Wild Type NSCLC. Nonetheless, TKIs are often recommended due to their acceptable toxicities. To address this treatment dilemma, the authors performed a systematic review and meta-analysis of randomized controlled trials, comparing first-generation EGFR TKIs (TARCEVA® and IRESSA®) treatment with conventional chemotherapy, in patients with advanced NSCLC, harboring Wild Type EGFR. This pooled analysis included 1605 patients from 11 clinical trials, with EGFR Wild Type NSCLC. The primary outcome measured was Progression Free Survival (PFS) and secondary outcomes were Objective Response Rate and Overall Survival. It was noted in this analysis that conventional chemotherapy was associated with longer PFS, compared with EGFR TKIs, among patients harboring Wild Type EGFR tumors. The authors noted that there was significant PFS benefit with chemotherapy, in trials using more sensitive EGFR mutation analysis platforms, than direct Sanger sequencing, and this may be the result of identifying the “true” Wild Type EGFR tumors. The objective response rate was higher at 16.8% with chemotherapy versus 7.2% for TKIs. There was however no statistically significant difference in the overall survival between the chemotherapy and TKI groups. When subgroups of patients in these trials were analyzed, outcomes were similar regardless of line of treatment, dominant ethinicity or EGFR mutation analysis method. The lack of improvement in Overall Survival in the chemotherapy group has been attributed to the large cross over rates in the trials that were analyzed. The authors concluded that conventional chemotherapy is associated with superior Progression Free Survival and Objective Response Rates, in patients with advanced NSCLC, harboring Wild Type EGFR tumors, compared with EGFR TKIs and the present guidelines recommending EGFR TKIs in this patient group has to be reevaluated. Lee J, Hahn S, Kim D, et al. JAMA 2014;311:1430-1437
In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced Non Small Cell Lung Cancer (NSCLC) cases with adenocarcinoma histology and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib) and IRESSA® (Gefitinib), changed the treatment paradigm in favor of targeted therapy, for this patient subset. It is estimated that approximately 10% of Western patient population and 50% of Asian patients with NSCLC, harbor EGFR activating mutations. EGFR Tyrosine Kinase Inhibitors have been shown to be superior to conventional chemotherapy in this patient group with improved Progression Free Survival (PFS) and Objective Response Rates. Patients with NSCLC should therefore be tested for the most common sensitizing mutations such as deletions in exon 19 and L858R point mutations in exon 21, as these patients clearly benefit from first line EGFR TKIs. EGFR expression by IHC (ImmunoHistoChemical) staining, EGFR gene copy by FISH (Fluorescence In Situ Hybridization) and blood based proteonomic testing by VERISTRAT® is currently not recommended for the selection of first line EGFR TKIs. There is presently no evidence indicating superiority of TKIs when compared with conventional chemotherapy for the second or third line treatment of EGFR Wild Type NSCLC. Nonetheless, TKIs are often recommended due to their acceptable toxicities. To address this treatment dilemma, the authors performed a systematic review and meta-analysis of randomized controlled trials, comparing first-generation EGFR TKIs (TARCEVA® and IRESSA®) treatment with conventional chemotherapy, in patients with advanced NSCLC, harboring Wild Type EGFR. This pooled analysis included 1605 patients from 11 clinical trials, with EGFR Wild Type NSCLC. The primary outcome measured was Progression Free Survival (PFS) and secondary outcomes were Objective Response Rate and Overall Survival. It was noted in this analysis that conventional chemotherapy was associated with longer PFS, compared with EGFR TKIs, among patients harboring Wild Type EGFR tumors. The authors noted that there was significant PFS benefit with chemotherapy, in trials using more sensitive EGFR mutation analysis platforms, than direct Sanger sequencing, and this may be the result of identifying the “true” Wild Type EGFR tumors. The objective response rate was higher at 16.8% with chemotherapy versus 7.2% for TKIs. There was however no statistically significant difference in the overall survival between the chemotherapy and TKI groups. When subgroups of patients in these trials were analyzed, outcomes were similar regardless of line of treatment, dominant ethinicity or EGFR mutation analysis method. The lack of improvement in Overall Survival in the chemotherapy group has been attributed to the large cross over rates in the trials that were analyzed. The authors concluded that conventional chemotherapy is associated with superior Progression Free Survival and Objective Response Rates, in patients with advanced NSCLC, harboring Wild Type EGFR tumors, compared with EGFR TKIs and the present guidelines recommending EGFR TKIs in this patient group has to be reevaluated. Lee J, Hahn S, Kim D, et al. JAMA 2014;311:1430-1437
Final overall survival analysis from the Cleopatra study of first-line pertuzumab, trastuzumab and docetaxel in patients with HER2-positive metastatic breast cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. Trastuzumab binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Despite this benefit, majority of these patients develop progressive disease within 18 months. The tumors in these patients continue to express HER2 although the lower sensitivity to HER2 targeted agents has been attributed to HER2 independent escape mechanisms. Treatment strategies for this patient population have included switching chemotherapy in subsequent lines of treatment and continuing HERCEPTIN®, combining another HER2 targeted agent, Lapatinib (TYKERB®) with Capecitabine (XELODA®) and dual HER2 inhibition with a combination of HERCEPTIN® and TYKERB®.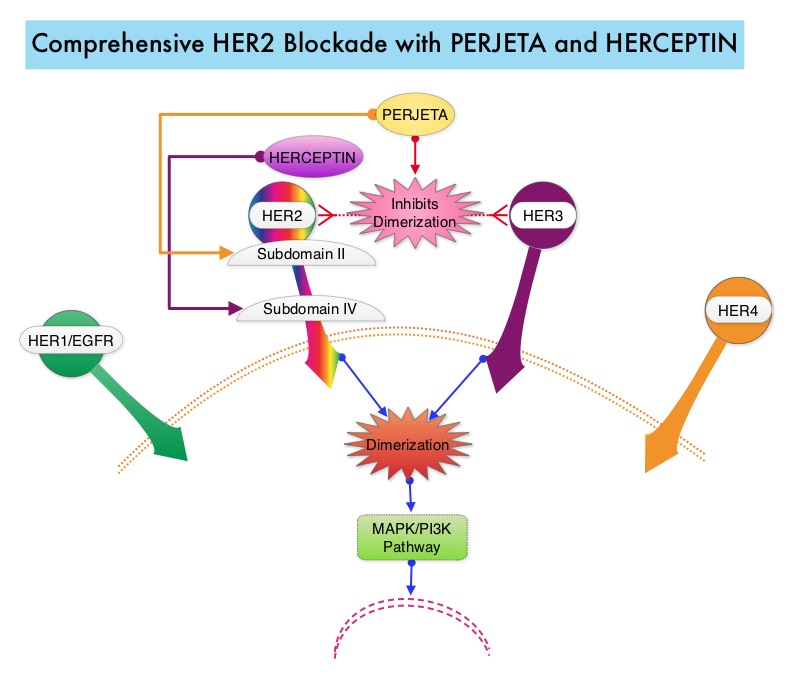 PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, objective response rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P=0.0002). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001) at the time of the final analysis. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer, should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Kim S, Cortes J, et al. Presented at: the 2014 Congress of the European Society of Medical Oncology; September 26-30, 2014; Madrid, Spain. Abstract 350O
PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, objective response rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P=0.0002). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001) at the time of the final analysis. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer, should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Kim S, Cortes J, et al. Presented at: the 2014 Congress of the European Society of Medical Oncology; September 26-30, 2014; Madrid, Spain. Abstract 350O
