SUMMARY: Cancer mortality rates in the United States have declined 20% from their peak of 215 per 100,000 in 1991 to 172 per 100,000 in 2010. With more than half a million Americans diagnosed with cancer each year, recent advances in cancer treatment and research has lead to improved survival and better quality of life, with 14.5 million cancer survivors alive in the US today. This Annual Report on Progress Against Cancer explored the clinical advances of the prior year (2014), that made the greatest impact on improving cancer care. This report was developed based on research published in peer-reviewed scientific and medical journals and information presented at major scientific meetings over a one year period between October 2013 to September 2014. A brief summary of this report (Part I) is presented. Part I includes, ADVANCE OF THE YEAR and ADVANCES IN PREVENTION AND SCREENING. Clinical trial details for several of these studies can be accessed at www.oncoprescribe.com
ADVANCE OF THE YEAR – TREATMENT OF CHRONIC LYMPHOCYTIC LEUKEMIA
Chronic Lymphocytic Leukemia (CLL) is the most common form of adult leukemia and is more common in the elderly. Four new therapies associated with fewer toxicities compared with standard therapy, were recently approved for patients with CLL.
Two Effective Treatment Options for Patients with Newly Diagnosed CLL
GAZYVA® (Obinutuzumab) is a fully humanized, third generation, type II, anti-CD20 monoclonal antibody that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. In a phase III trial involving 589 treatment naïve CLL patients, GAZYVA® in combination with LEUKERAN® (Chlorambucil) more than doubled the Progression Free Survival (PFS) from 11.1 months with LEUKERAN® alone to 26.7 months (HR=0.18, P<0.001). The combination of GAZYVA® and LEUKERAN® also prolonged Overall Survival (OS) when compared to LEUKERAN® alone (HR=0.41; P=0.002). This benefit however was not noted with the RITUXAN® plus LEUKERAN® combination. Treatment with GAZYVA® plus LEUKERAN® when compared with RITUXAN® plus LEUKERAN® resulted in a longer PFS (26.7 vs15.2 months; HR=0.39; P<0.001), higher complete response rates (20.7% vs 7.0%) and deeper molecular responses.
ARZERRA® (Ofatumumab), a second generation fully human IgG 1 monoclonal antibody, which targets a different region (different epitope) of the CD20 molecule in combination with LEUKERAN®, was compared with LEUKERAN® alone, as first line treatment in a study involving 447 CLL patients. The median PFS was 22.4 months for patients receiving ARZERRA® in combination with LEUKERAN® compared with 13.1 months for those receiving single agent LEUKERAN® (HR=0.57, P< 0.001). The Objective Response Rate was higher with the combination regimen versus single agent LEUKERAN® (82% vs 69%, P=0.001).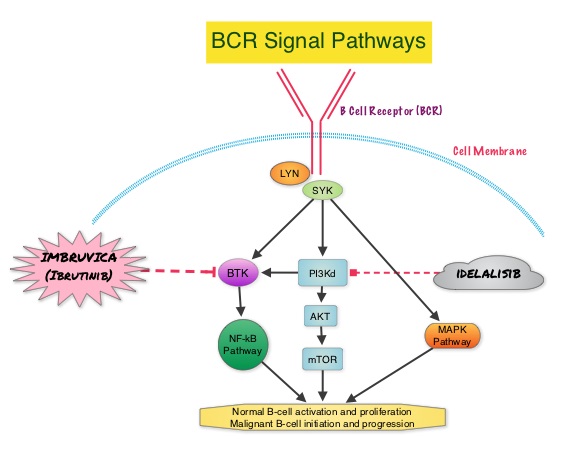
Two New Non-Chemotherapy Alternatives for Relapsed and Treatment-Resistant CLL
IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). IMBRUVICA® was compared to single agent ARZERRA® in a phase III trial involving 391 patients with relapsed CLL. Thirty percent (30%) of the patients had deletion of chromosome 17p. At a median follow up of 9.4 months, IMBRUVICA® significantly prolonged PFS compared to ARZERRA® (median not reached vs 8.1 months; HR 0.215, P<0.0001) with a 78.5% reduction in the risk of disease progression and also significantly improved OS (median not reached, HR 0.43, P=0.0049) when compared with ARZERRA®, with a 57% reduction in the risk of death.
In a phase III study involving 220 previously treated patients with recurrent CLL, ZYDELIG® (Idelalisib), a highly selective oral inhibitor of the enzyme PhosphoInositide 3-Kinase (PI3K) that specifically blocks the delta isoform of PI3K enzyme and its signaling pathway, was combined with RITUXAN® and compared with placebo given along with RITUXAN®. The median PFS with ZYDELIG® in combination with RITUXAN® was significantly prolonged compared with Placebo and RITUXAN® (10.7 months vs 5.5 months). An improvement in the Overall Survival (OS) was also noted in the ZYDELIG® group compared with patients in the RITUXAN® and placebo group (HR = 0.28; P = 0.018).
ADVANCES IN PREVENTION AND SCREENING
Breast Cancer Prevention
The only two drugs currently approved by the FDA to prevent breast cancer are NOLVADEX® (Tamoxifen) and EVISTA® (Raloxifene). These agents block the estrogen receptors and can be used in both pre and postmenopausal women. NOLVADEX® is however associated with thromboembolic evens as well as endometrial carcinoma. ARIMIDEX® (Anastrozole), an Aromatase Inhibitor (AI), in a randomized, double blind, placebo controlled trial, involving 3864 women at increased risk of breast cancer, reduced this risk of breast cancer by 53% compared to placebo, over a 5-year period (P<0.0001), in post-menopausal women. This benefit with ARIMIDEX® was accomplished without increase in the risk of heart attacks or fractures, compared with placebo. There was however increase in the incidence of joint and muscle pain as well as hot flushes and night sweats. Another AI, AROMASIN® (Exemestane) in a previously published study (MAP.3 trial) significantly reduced the incidence of all breast cancers by 53% and invasive breast cancers by 65%, after a median follow up of 3 years.
Screening for Lung Cancer
The United States Preventive Services Task Force (USPSTF) recommended annual screening for lung cancer with Low Dose Computed Tomography in adult individuals, between ages 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. The use of Low Dose CT (LDCT) scans for 3 years in this high risk, healthy patients, resulted in a 20% reduction in Lung cancer mortality, compared to screening with a chest X-Ray in the NCI-sponsored National Lung Screening Trial (NLST).
Masters GA, Krilov L, Bailey HH, et al. Published online before print January 20, 2015, doi: 10.1200/JCO.2014.59.9746

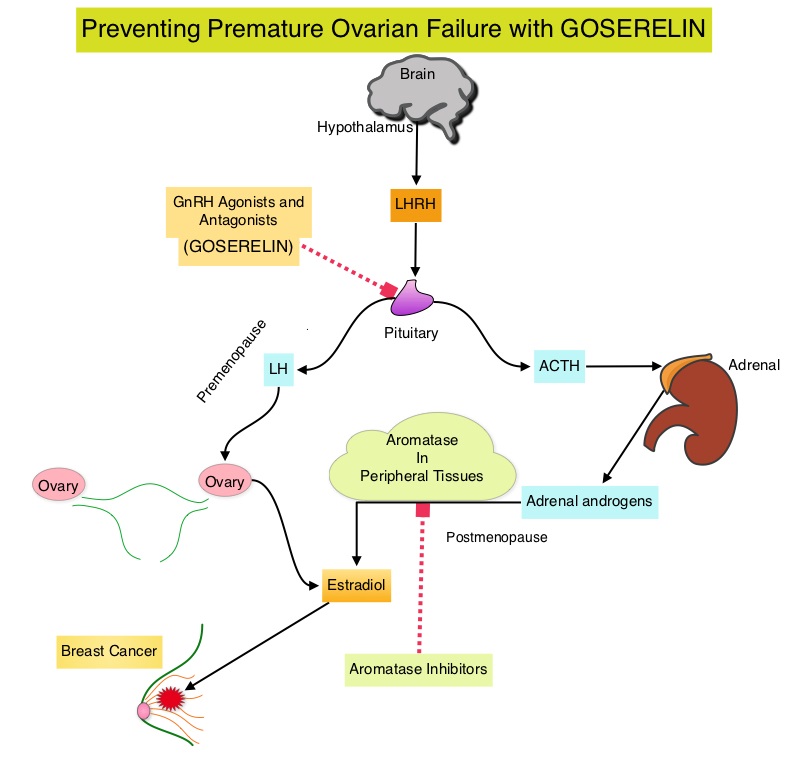 Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone receptor negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. The median age of the patients was 38 years and median follow up was 4.1 years. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.04). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of Premature Ovarian Failure with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Goserelin for Ovarian Protection during Breast-Cancer Adjuvant Chemotherapy. Moore HC, Unger JM, Phillips K, et al. N Engl J Med 2015; 372:923-932
Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone receptor negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. The median age of the patients was 38 years and median follow up was 4.1 years. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.04). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of Premature Ovarian Failure with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Goserelin for Ovarian Protection during Breast-Cancer Adjuvant Chemotherapy. Moore HC, Unger JM, Phillips K, et al. N Engl J Med 2015; 372:923-932
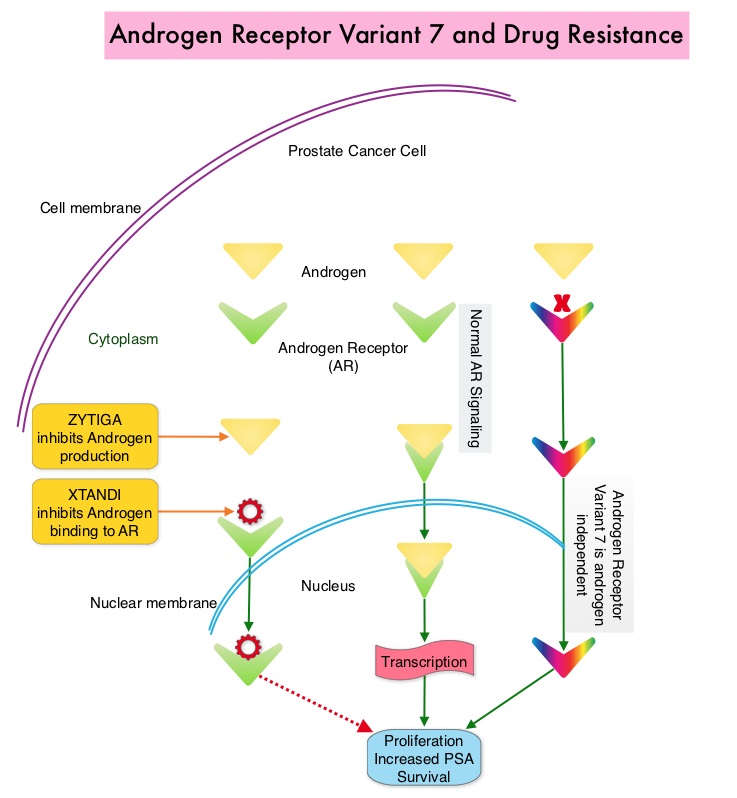 Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)
Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)
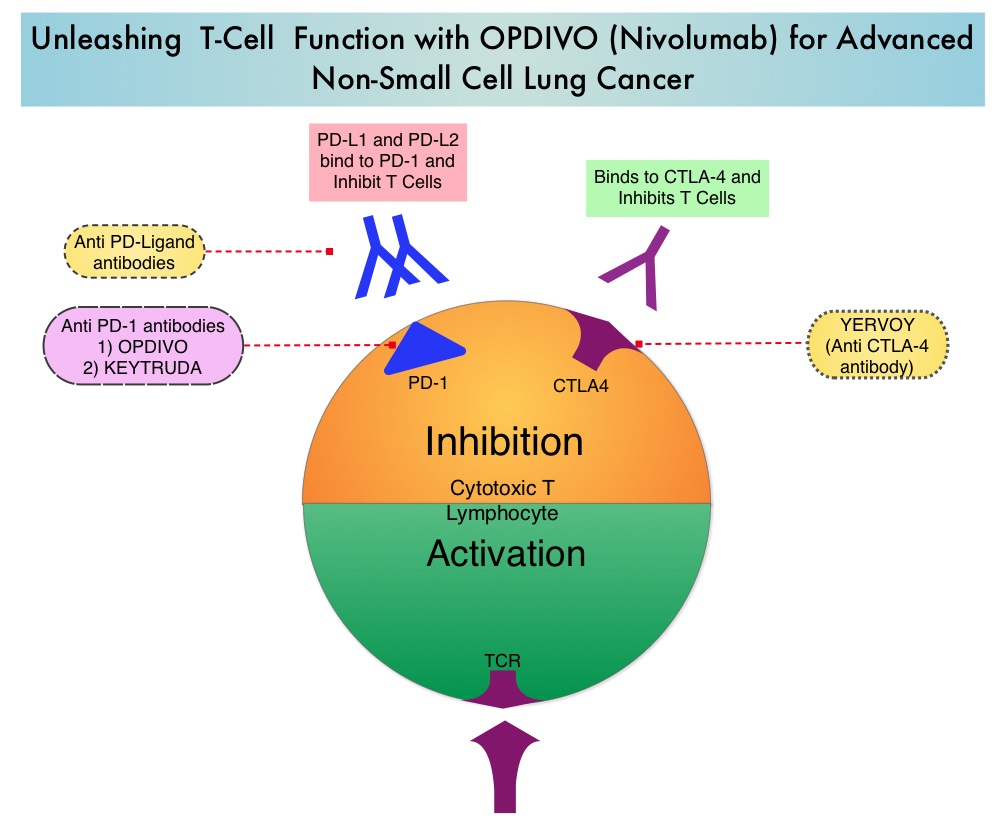 The development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints has however changed the treatment paradigm. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The approval of OPDIVO® was based on CheckMate-017, an open label, multicenter, multinational randomized phase III trial in which 272 patients with metastatic squamous NSCLC who had experienced disease progression during or after one prior platinum-based chemotherapy regimen were randomized to receive OPDIVO® (Nivolumab) 3 mg/kg IV every 2 weeks (N=135) or TAXOTERE® (Docetaxel) 75 mg/m2 IV every 3 weeks (N=137). The primary endpoint was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS) and Objective Response Rate (ORR). This study was stopped early at the protocol pre-specified interim analysis after an independent monitoring panel determined that the primary endpoint of improved Overall Survival (OS) with OPDIVO® had been reached. The median OS was 9.2 months for patients assigned to OPDIVO® and 6 months for those in the TAXOTERE® group (HR=0.59; P=0.00025). This suggested a 41% improvement in the OS with OPDIVO® compared to TAXOTERE®. This FDA approval was further supported by a single arm, multinational, multicenter trial in patients with metastatic squamous NSCLC (N=117) who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. OPDIVO® in this study, was administered as a single agent at 3mg/kg IV every two weeks until disease progression or treatment discontinuation. The primary endpoint was Objective Response Rate (ORR) and exploratory endpoints were Overall Survival (OS), Progression Free Survival (PFS) and efficacy, based on PD-L1 expression status. With 11 months of minimum follow up, the Objective Response Rate (ORR) was 15% independent of PD-L1 status. The estimated one-year survival rate was 41% and median Overall Survival was 8.2 months. The authors noted that an additional 26% of patients had stable disease for a median duration of 6 months, resulting in a disease control rate (ORR+stable disease) of 41%. The most frequent grade 3-4 adverse events noted in at least 5% of the patients were fatigue, dyspnea and musculoskeletal pain. OPDIVO® will now be a new treatment option, with survival advantage, for patients with advanced relapsed and refractory metastatic squamous NSCLC. Phase II study of nivolumab (Anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer. Ramalingam SS, Mazieres J, Planchard D, et al. Presented at: 2014 Multidisciplinary Symposium in Thoracic Oncology; Chicago, IL. LBA#3462
The development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints has however changed the treatment paradigm. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The approval of OPDIVO® was based on CheckMate-017, an open label, multicenter, multinational randomized phase III trial in which 272 patients with metastatic squamous NSCLC who had experienced disease progression during or after one prior platinum-based chemotherapy regimen were randomized to receive OPDIVO® (Nivolumab) 3 mg/kg IV every 2 weeks (N=135) or TAXOTERE® (Docetaxel) 75 mg/m2 IV every 3 weeks (N=137). The primary endpoint was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS) and Objective Response Rate (ORR). This study was stopped early at the protocol pre-specified interim analysis after an independent monitoring panel determined that the primary endpoint of improved Overall Survival (OS) with OPDIVO® had been reached. The median OS was 9.2 months for patients assigned to OPDIVO® and 6 months for those in the TAXOTERE® group (HR=0.59; P=0.00025). This suggested a 41% improvement in the OS with OPDIVO® compared to TAXOTERE®. This FDA approval was further supported by a single arm, multinational, multicenter trial in patients with metastatic squamous NSCLC (N=117) who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. OPDIVO® in this study, was administered as a single agent at 3mg/kg IV every two weeks until disease progression or treatment discontinuation. The primary endpoint was Objective Response Rate (ORR) and exploratory endpoints were Overall Survival (OS), Progression Free Survival (PFS) and efficacy, based on PD-L1 expression status. With 11 months of minimum follow up, the Objective Response Rate (ORR) was 15% independent of PD-L1 status. The estimated one-year survival rate was 41% and median Overall Survival was 8.2 months. The authors noted that an additional 26% of patients had stable disease for a median duration of 6 months, resulting in a disease control rate (ORR+stable disease) of 41%. The most frequent grade 3-4 adverse events noted in at least 5% of the patients were fatigue, dyspnea and musculoskeletal pain. OPDIVO® will now be a new treatment option, with survival advantage, for patients with advanced relapsed and refractory metastatic squamous NSCLC. Phase II study of nivolumab (Anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer. Ramalingam SS, Mazieres J, Planchard D, et al. Presented at: 2014 Multidisciplinary Symposium in Thoracic Oncology; Chicago, IL. LBA#3462
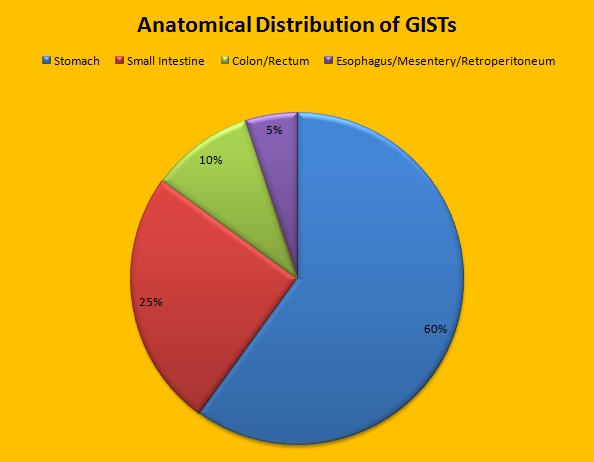 Approximately 60% of the primary GISTs originate in the stomach, 25% in the small intestine, 10% in the colon and rectum and the rest at other sites such as the esophagus, mesentery and retroperitoneum. GISTs were misclassified as leiomyomas or leiomyosarcomas until 1998, when the discovery of mutations in the c-KIT oncogene, lead to a better understanding of these tumors. C-KIT oncogene encodes the transmembrane KIT receptor tyrosine kinase. Approximately 85% of the GISTs have activating (gain of function) KIT mutations and are positive for the CD117 antigen, an epitope of KIT receptor tyrosine kinase. Positive CD117 by ImmunoHistoChemistry (IHC) is however not specific for GIST, as weak reactivity occurs with other mesenchymal tumors. IHC staining for protein kinase C theta and DOG1 are helpful in distinguishing GIST from other mesenchymal tumors, particularly those that are KIT-negative. DOG1 (Discovered On GIST 1) is a protein of unknown function that is expressed strongly in GISTs and is rarely expressed in other mesenchymal neoplasms. KIT mutations in GISTs occur in Exon 9 (10%), Exon 11 (67%), Exon 13 (1%) and Exon 17 (1%). About 5% of the GISTs have activating mutation in the Platelet-Derived Growth Factor Receptor alpha (PDGFRA) gene which encodes for another tyrosine kinase receptor. Approximately 10% to 20% of GISTs have no KIT or PDGFRA mutations and are referred as wild-type GISTs. Mutational status is important as they may predict response to GLEEVEC® and also have prognostic significance. Patients with KIT exon 11 mutations are most sensitive and have a much higher response to GLEEVEC® whereas those with KIT exon 9 mutation or wild-type c-KIT may require a higher dose of GLEEVEC® (800 mg daily dose). KIT exon 11 mutations affecting the codons 557 and 558 is an independent adverse prognostic factor and associated with higher incidence of metastases whereas GISTs with KIT exon 9 mutations usually arise in the small bowel and are associated with frequent recurrences. GISTs with PDGFRA mutations in general have low mitotic count and low malignant potential and those with PDGFRA exon 18 mutation have favorable survival outcomes and located in the stomach. They are however resistant to GLEEVEC®. Although mutated KIT and PDGFRA have been identified as important driver mutations for GIST oncogenesis, the clinical significance of their single mutations has been unclear. To address this, the authors in this study identified 3067 patients with GIST from databases who had macroscopically complete tumor excision and had no detectable metastases at the time of diagnosis. Information on mutation analysis was available on 1505 tumors. The researchers then analyzed associations between KIT and PDGFRA mutations and Recurrence Free Survival (RFS) in this patient population treated with surgery alone.
Approximately 60% of the primary GISTs originate in the stomach, 25% in the small intestine, 10% in the colon and rectum and the rest at other sites such as the esophagus, mesentery and retroperitoneum. GISTs were misclassified as leiomyomas or leiomyosarcomas until 1998, when the discovery of mutations in the c-KIT oncogene, lead to a better understanding of these tumors. C-KIT oncogene encodes the transmembrane KIT receptor tyrosine kinase. Approximately 85% of the GISTs have activating (gain of function) KIT mutations and are positive for the CD117 antigen, an epitope of KIT receptor tyrosine kinase. Positive CD117 by ImmunoHistoChemistry (IHC) is however not specific for GIST, as weak reactivity occurs with other mesenchymal tumors. IHC staining for protein kinase C theta and DOG1 are helpful in distinguishing GIST from other mesenchymal tumors, particularly those that are KIT-negative. DOG1 (Discovered On GIST 1) is a protein of unknown function that is expressed strongly in GISTs and is rarely expressed in other mesenchymal neoplasms. KIT mutations in GISTs occur in Exon 9 (10%), Exon 11 (67%), Exon 13 (1%) and Exon 17 (1%). About 5% of the GISTs have activating mutation in the Platelet-Derived Growth Factor Receptor alpha (PDGFRA) gene which encodes for another tyrosine kinase receptor. Approximately 10% to 20% of GISTs have no KIT or PDGFRA mutations and are referred as wild-type GISTs. Mutational status is important as they may predict response to GLEEVEC® and also have prognostic significance. Patients with KIT exon 11 mutations are most sensitive and have a much higher response to GLEEVEC® whereas those with KIT exon 9 mutation or wild-type c-KIT may require a higher dose of GLEEVEC® (800 mg daily dose). KIT exon 11 mutations affecting the codons 557 and 558 is an independent adverse prognostic factor and associated with higher incidence of metastases whereas GISTs with KIT exon 9 mutations usually arise in the small bowel and are associated with frequent recurrences. GISTs with PDGFRA mutations in general have low mitotic count and low malignant potential and those with PDGFRA exon 18 mutation have favorable survival outcomes and located in the stomach. They are however resistant to GLEEVEC®. Although mutated KIT and PDGFRA have been identified as important driver mutations for GIST oncogenesis, the clinical significance of their single mutations has been unclear. To address this, the authors in this study identified 3067 patients with GIST from databases who had macroscopically complete tumor excision and had no detectable metastases at the time of diagnosis. Information on mutation analysis was available on 1505 tumors. The researchers then analyzed associations between KIT and PDGFRA mutations and Recurrence Free Survival (RFS) in this patient population treated with surgery alone.