SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 231,840 new cases of invasive breast cancer will be diagnosed in 2015 and over 40,000 women will die of the disease. Taxanes, which include TAXOL® (Paclitaxel) and TAXOTERE® (Docetaxel) have an important role in the treatment of breast cancer and have been shown in numerous clinical studies to improve survival in patients with early-stage breast cancer and select group of patients with metastatic breast cancer.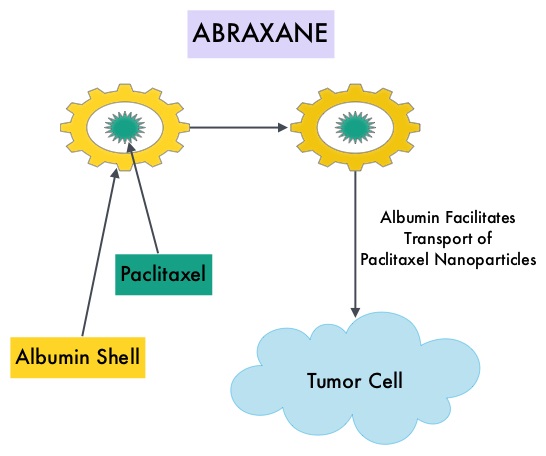 ABRAXANE® (Nab-paclitaxel) is a solvent free, albumin-encapsulated nanoparticle formulation of the taxane, Paclitaxel. By virtue of its formulation, unlike TAXOL®, hypersensitivity reactions are uncommon with ABRAXANE® and can therefore be rapidly infused and premedications are not needed. Further, higher tumor drug concentrations are achieved with ABRAXANE® compared to conventional Paclitaxel (TAXOL®). Previously published studies have shown that ABRAXANE® is superior to TAXOL® in metastatic breast cancer. GeparSepto trial is a randomized phase III study in which weekly ABRAXANE® was compared head to head with weekly TAXOL® in a neoadjuvant setting. This study enrolled 1204 treatment naïve, high risk patients, with clinical T2-T4d invasive breast carcinoma. Eligible patients included those with unilateral, bilateral, operable or inoperable breast cancer. The median age was 49 years, median tumor size was 3 cm, 23% of the patients had triple-negative disease and 33% had HER-2 positive tumors. Patients were randomized 1:1 to receive either ABRAXANE® 125 mg/m2 IV or TAXOL® 80 mg/m2 IV, weekly for 12 weeks followed by 4 cycles of Epirubicin 90 mg/m2 IV and Cyclophosphamide 600 mg/m2 IV. Patients with HER-2 positive tumors also received HERCEPTIN® (Trastuzumab) and PERJETA® (Pertuzumab). The primary endpoint was pathologic Complete Response (pCR), defined as absence of microscopic residual invasive or noninvasive viable tumor cells in all resected specimens of the breast and axilla. This endpoint was chosen because pathologic Complete Response serves as a surrogate marker for long-term efficacy and outcomes. In this trial, the researchers noted a pathologic Complete Response rate of 38% with ABRAXANE® compared to 29% with TAXOL® (P<0.01). On subgroup analysis, this benefit was even more evident in patients with triple negative breast cancer (N=275), with a pCR rate of 48.2% in the ABRAXANE® group compared with 25.7% in the TAXOL® group (P <0.001). The incidence of peripheral neuropathy was higher in the ABRAXANE® group compared to TAXOL® group and this was attributed to higher weekly doses of ABRAXANE® administered. It is felt that a lower dose of weekly ABRAXANE® (100 mg/m2) would result in a decrease in the incidence of peripheral neuropathy, without compromising efficacy. The authors concluded that ABRAXANE® is superior to TAXOL® in early stage, high risk patients with breast cancer and this benefit is even more evident in those patients with triple negative disease, which comprises about 15% of all breast cancers. Untch M, Jackisch C, Schneeweiss A, et al. A randomized phase III trial comparing neoadjuvant chemotherapy with weekly nanoparticle-based paclitaxel with solvent-based paclitaxel followed by anthracyline/cyclophosphamide for patients with early breast cancer (GeparSepto); GBG 69. Paper presented at: 2014 San Antonio Breast Cancer Symposium; December 9-13, 2014; San Antonio, TX. Abstract S2-07.
ABRAXANE® (Nab-paclitaxel) is a solvent free, albumin-encapsulated nanoparticle formulation of the taxane, Paclitaxel. By virtue of its formulation, unlike TAXOL®, hypersensitivity reactions are uncommon with ABRAXANE® and can therefore be rapidly infused and premedications are not needed. Further, higher tumor drug concentrations are achieved with ABRAXANE® compared to conventional Paclitaxel (TAXOL®). Previously published studies have shown that ABRAXANE® is superior to TAXOL® in metastatic breast cancer. GeparSepto trial is a randomized phase III study in which weekly ABRAXANE® was compared head to head with weekly TAXOL® in a neoadjuvant setting. This study enrolled 1204 treatment naïve, high risk patients, with clinical T2-T4d invasive breast carcinoma. Eligible patients included those with unilateral, bilateral, operable or inoperable breast cancer. The median age was 49 years, median tumor size was 3 cm, 23% of the patients had triple-negative disease and 33% had HER-2 positive tumors. Patients were randomized 1:1 to receive either ABRAXANE® 125 mg/m2 IV or TAXOL® 80 mg/m2 IV, weekly for 12 weeks followed by 4 cycles of Epirubicin 90 mg/m2 IV and Cyclophosphamide 600 mg/m2 IV. Patients with HER-2 positive tumors also received HERCEPTIN® (Trastuzumab) and PERJETA® (Pertuzumab). The primary endpoint was pathologic Complete Response (pCR), defined as absence of microscopic residual invasive or noninvasive viable tumor cells in all resected specimens of the breast and axilla. This endpoint was chosen because pathologic Complete Response serves as a surrogate marker for long-term efficacy and outcomes. In this trial, the researchers noted a pathologic Complete Response rate of 38% with ABRAXANE® compared to 29% with TAXOL® (P<0.01). On subgroup analysis, this benefit was even more evident in patients with triple negative breast cancer (N=275), with a pCR rate of 48.2% in the ABRAXANE® group compared with 25.7% in the TAXOL® group (P <0.001). The incidence of peripheral neuropathy was higher in the ABRAXANE® group compared to TAXOL® group and this was attributed to higher weekly doses of ABRAXANE® administered. It is felt that a lower dose of weekly ABRAXANE® (100 mg/m2) would result in a decrease in the incidence of peripheral neuropathy, without compromising efficacy. The authors concluded that ABRAXANE® is superior to TAXOL® in early stage, high risk patients with breast cancer and this benefit is even more evident in those patients with triple negative disease, which comprises about 15% of all breast cancers. Untch M, Jackisch C, Schneeweiss A, et al. A randomized phase III trial comparing neoadjuvant chemotherapy with weekly nanoparticle-based paclitaxel with solvent-based paclitaxel followed by anthracyline/cyclophosphamide for patients with early breast cancer (GeparSepto); GBG 69. Paper presented at: 2014 San Antonio Breast Cancer Symposium; December 9-13, 2014; San Antonio, TX. Abstract S2-07.
Month: April 2015
Indolent Non-Follicular B-Cell Lymphoma Treatment Guidelines
SUMMARY: Indolent Non-Follicular B-Cell Lymphoma (INFBCL) are mature B cell lymphoproliferative disorders and include Nodal Marginal Zone Lymphoma (NMZL), Extranodal Marginal Zone Lymphoma (ENMZL) of Mucosa-Associated Lymphoid Tissue (MALT) lymphoma, Splenic Marginal Zone Lymphoma (SMZL), LymphoPlasmacytic Lymphoma (LPL) and Small Lymphocytic Lymphoma (SLL).
NMZL, LPL, and SLL
Recommendations for Diagnosis:
1) Excision biopsy of material from the primary disease site (ie, lymph node)
2) Fine Needle Aspiration biopsy is not recommended for the diagnosis or sub-typing. Computed Tomography (CT)-guided core biopsy can be an alternative diagnostic approach when thoracotomy or laparotomy are needed for lymph node biopsy.
3) A definitive diagnosis of LPL can only be made using bone marrow material.
Recommendations for Staging and Pretreatment Evaluation:
1) Complete history and physical examination, assessing Performance Status and B symptoms
2) Lab tests including CBC, CMP, LDH, B2-Microglobulin, hemolysis workup if anemic, SPEP, UPEP with immunofixation, serology for Hepatitis B,C and HIV, Flow Cytometry if peripheral blood shows abnormal lymphocytes or absolute lymphocytosis present and bone marrow aspirate and biopsy
3) CT of the neck, chest, abdomen, and pelvis (FDG-PET is not routinely indicated for this group of lymphomas as the avidity of FDG uptake is lower)
4) In LPL with monoclonal protein in the urine or serum, a fat pad biopsy for Congo red staining and an ultrasound cardiac scan are recommended when Amyloidosis is suspected
5) ECHO or MUGA scan, if treatment with anthracyclines are planned
6) Pregnancy testing in women of child-bearing age and counseling for the preservation of fertility
Recommendations regarding treatment initiation
1) Initiation of Chemotherapy or Immunotherapy should be based on the identification of symptoms as defined by the GELF or BNLI criteria.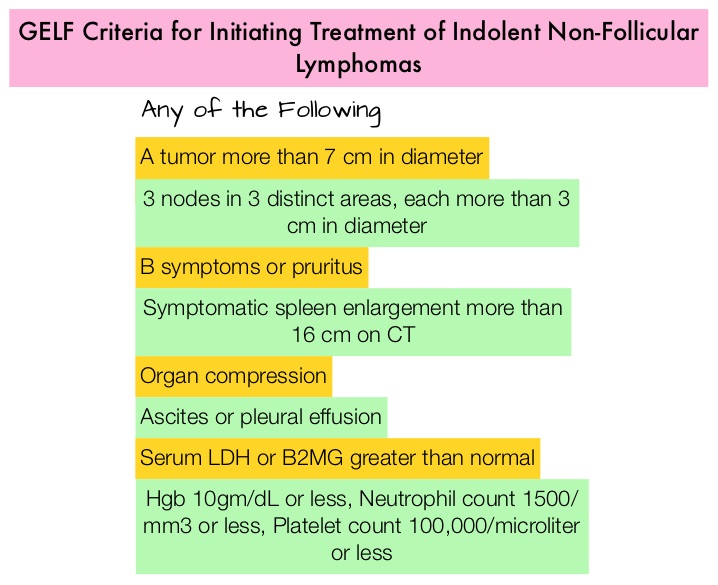
2) Monoclonal protein-related symptoms such as hyperviscosity syndrome, renal failure, vasculitis and a lymphocyte doubling time of less than 6 months in those with leukemia, are indications for treatment.
3) Chemoimmunotherapy or Chemotherapy alone as early treatment is not recommended in asymptomatic advanced disease and observation alone is recommended in patients who do not meet the criteria for treatment with Chemotherapy or Immunotherapy
Recommendations for First Line Therapy
1) Patients with stage I or nonbulky stage II disease should be promptly treated with involved field Radiotherapy (RT) with or without chemotherapy. Observation alone may be a reasonable alternative if the potential toxicity of RT outweighs the potential benefits, particularly in elderly patients or if the patient refuses RT.
2) FLUDARA® (Fludarabine) plus RITUXAN® (Rituximab) – FR or TREANDA® (Bendamustine) plus RITUXAN® (BR) is the recommended first line therapy.
3) The combination of FLUDARA®, Cyclophosphamide and RITUXAN® (FCR) should be recommended to patients less than 70 years of age without significant comorbidities, and TREANDA® plus RITUXAN® should be preferred for patients 70 years or older with significant comorbidities.
Recommendations for Postinduction Therapy
Maintenance RITUXAN® is not recommended and patients with a partial response (PR) with no symptoms or signs of active disease, should be observed without additional treatment until progression.
Recommendations for Patients with Relapse
BR combination is the preferred treatment and new combinations of RITUXAN® with REVLIMID® (Lenalidomide) or VELCADE® (Bortezomib) should be considered experimental
ENMZL of MALT Lymphoma of Gastric Mucosa (Gastric MALToma)
MALT lymphoma histology is the most frequent among Marginal Zone Lymphomas and the most common extranodal MALT lymphoma involves the gastrointestinal tract.
Recommendations for Diagnosis:
1) Biopsy of material from the primary disease site
2) A molecular genetic analysis of lymphoma tissue for the detection of t(11;18) is recommended to identify disease that is unlikely to respond to antibiotic therapy
3) The presence of active Helicobacter pylori (Hp) infection must be determined using histologic testing. In the case of negative results, serology testing, stool antigen testing, or the urea breath test is recommended
Recommendations for Staging and Pretreatment Evaluation:
1) A detailed description of the extent of gastric lesions, gastric wall infiltration, and involvement of the perigastric lymph nodes, with ultrasound-guided endoscopy.
2) Along with the conventional staging procedures, additional investigations should include CT of the neck, chest, abdomen and pelvis and bone marrow biopsy
3) Staging classification should use the Ann Arbor staging system modified according to the Paris staging system
Recommendations for First Line Therapy
1) The first-line treatment of Hp-positive patients with gastric MALT lymphoma is Hp eradication therapy, independent of the disease stage. Surgery should only be considered for patients with perforation or bleeding, not amenable to endoscopy
2) Patients with Hp-negative localized gastric MALT lymphoma can also be treated with eradication therapy although the chance of a response is low and disease status should be closely monitored during therapy
3) After successful Hp eradication, consolidation chemotherapy is not indicated
Recommendations for non-responding and relapsed Patients
1) Radiation therapy is recommended for patients with stage IE-IIE gastric MALT lymphoma
2) RITUXAN® plus chemotherapy is recommended for those with other disease stages
SMZL (Splenic Lymphoma With Circulating Villous Lymphocytes)
Recommendations for Diagnosis:
1) SMZL can be diagnosed by splenectomy and examination of the splenic tissue
2) SMZL can also be diagnosed by a combination of bone marrow biopsy and an immunocytochemistry profile (intrasinusoidal infiltration by CD20+ cells), peripheral blood and bone marrow aspirate morphology as well as flow cytometry.
Recommendations for Staging and Pretreatment Evaluation:
1) Complete history and physical examination assessing performance status and B symptoms
2) Lab tests should include CBC, CMP, LDH, B2-Microglobulin, hemolysis workup if anemic, SPEP, UPEP with immunofixation, cryoglobulin and cryocrit. Serology for Hepatitis B,C and HIV, flow cytometry if peripheral blood shows abnormal lymphocytes or absolute lymphocytosis present and bone marrow aspirate and biopsy
3) CT of the neck, chest, abdomen, and pelvis (FDG-PET is not routinely indicated for this group of lymphomas as the avidity of FDG uptake is lower)
4) Pregnancy testing in women of child-bearing age and counseling for the preservation of fertility
Recommendations regarding treatment initiation
Patients with SMZL should be initiated on therapy if there is progressive or symptomatic splenomegaly, hemoglobin less than 10 g/dL, neutrophils less than 1000/μL, progressive thrombocytopenia, systemic symptoms, progressive nodal disease and autoimmune hemolytic anemia.
Recommendations for First Line Therapy
1) Patients who are HCV positive with no indications for anti-lymphoma therapy, should be treated for HCV infection.
2) For those requiring therapy for lymphoma, the options include splenectomy, chemotherapy alone, RITUXAN® alone, or chemoimmunotherapy. Chemoimmunotherapy is indicated for good PS patients with disseminated disease. Combinations therapies with proven efficacy include RITUXAN® in combination with Chlorambucil, CVP (Cyclophosphamide, Vincristine, Prednisone), FLUDARA® or 2-CDA (Cladribine).
3) Splenectomy should be recommended when patients present with splenomegaly-related cytopenias in the absence of a high percentage of leukemic cells in the peripheral blood, heavy bone marrow infiltration, and diffuse nodal disease. In patients who are HCV positive, splenectomy should be considered only after the exclusion of a severe chronic liver disease.
4) Single agent RITUXAN® may be considered for patients without disseminated disease and for patients with contraindications to surgery or chemoimmunotherapy.
Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation Guidelines for the Management of Indolent, Nonfollicular B-Cell Lymphoma (Marginal Zone, Lymphoplasmacytic, and Small Lymphocytic Lymphoma). Tarella C, Arcaini L, Baldini L, et al. Clinical Lymphoma, Myeloma & Leukemia. 2015; 15:75–85
Platelet Transfusions Detrimental in TTP and HIT
SUMMARY: Patients with Thrombotic Thrombocytopenic Purpura (TTP), Heparin Induced Thrombocytopenia (HIT) and Immune Thrombocytopenic Purpura (ITP) often receive prophylactic platelet transfusions even though there are no data to support this practice. These transfusions are often given preemptively to reduce the risk for spontaneous bleeding in patients who are thrombocytopenic. Thrombocytopenia refers to a platelet count below the lower limit of the normal range used by the laboratory performing the count. In the United States, a little over 2 million platelet units are transfused annually. The known risks associated with platelet transfusion include febrile and allergic reactions, Transfusion Related Acute Lung Injury and infections. The authors in this study utilized a Nationwide Inpatient Sample of patients over a 5 year period from 2007-2011and evaluated the risks associated with platelet transfusions.
The known risks associated with platelet transfusion include febrile and allergic reactions, Transfusion Related Acute Lung Injury and infections. The authors in this study utilized a Nationwide Inpatient Sample of patients over a 5 year period from 2007-2011and evaluated the risks associated with platelet transfusions.  They identified 10,624 hospitalizations with TTP and platelet transfusions were reported in 10.1%, 6,332 hospitalizations with HIT and 7.1% received platelet transfusions and 79,980 admissions with ITP and 25.8% received platelet transfusions. The Odds Ratio (OR) was calculated after adjusting for age, gender, clinical severity and acuity. (An Odds Ratio is a measure of association between an exposure and an outcome. The OR represents the odds that an outcome will occur given a particular exposure, compared to the odds of the outcome occurring in the absence of that exposure). The researchers noted some alarming findings. Platelet transfusions in TTP were associated with higher odds of arterial thrombosis (OR=5.8), Acute Myocardial Infarction (OR=2.0) and mortality (OR=2.0). Platelet transfusions in HIT were associated with higher odds of arterial thrombosis (OR=3.4) and mortality (OR=5.2). Platelet transfusions in TTP or HIT however, was not associated with venous thrombosis. There were no increased risks among patients with ITP, who received platelet transfusions. The authors concluded that until future trials provide additional information, platelet transfusions should be considered a relative contraindication in patients with TTP and HIT and should only be used in situations where severe or potentially life threatening bleeding is refractory to other therapies. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Goel R, Ness PM, Takemoto CM, et al. Blood. 2015;125:1470-1476.
They identified 10,624 hospitalizations with TTP and platelet transfusions were reported in 10.1%, 6,332 hospitalizations with HIT and 7.1% received platelet transfusions and 79,980 admissions with ITP and 25.8% received platelet transfusions. The Odds Ratio (OR) was calculated after adjusting for age, gender, clinical severity and acuity. (An Odds Ratio is a measure of association between an exposure and an outcome. The OR represents the odds that an outcome will occur given a particular exposure, compared to the odds of the outcome occurring in the absence of that exposure). The researchers noted some alarming findings. Platelet transfusions in TTP were associated with higher odds of arterial thrombosis (OR=5.8), Acute Myocardial Infarction (OR=2.0) and mortality (OR=2.0). Platelet transfusions in HIT were associated with higher odds of arterial thrombosis (OR=3.4) and mortality (OR=5.2). Platelet transfusions in TTP or HIT however, was not associated with venous thrombosis. There were no increased risks among patients with ITP, who received platelet transfusions. The authors concluded that until future trials provide additional information, platelet transfusions should be considered a relative contraindication in patients with TTP and HIT and should only be used in situations where severe or potentially life threatening bleeding is refractory to other therapies. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Goel R, Ness PM, Takemoto CM, et al. Blood. 2015;125:1470-1476.
Higher Serum Vitamin D Levels May Improve Survival in Patients with Advanced Colon Cancer
SUMMARY: The American Cancer Society estimates that approximately 133,000 new cases of colorectal cancer will be diagnosed in the United States in 2015 and close to 50,000 are expected to die of the disease. There is a growing body of evidence suggesting that Vitamin D has colon cancer preventing properties and may induce antitumor immunity. A recent study by Song and colleagues (Gut. 2015;64:260-271) showed that high plasma level 25-Hydroxy Vitamin D [25(OH)D] was associated with lower risk of colorectal cancer with intense immune reaction, supporting that vitamin D through tumor-host interaction may play a role in cancer immunoprevention. 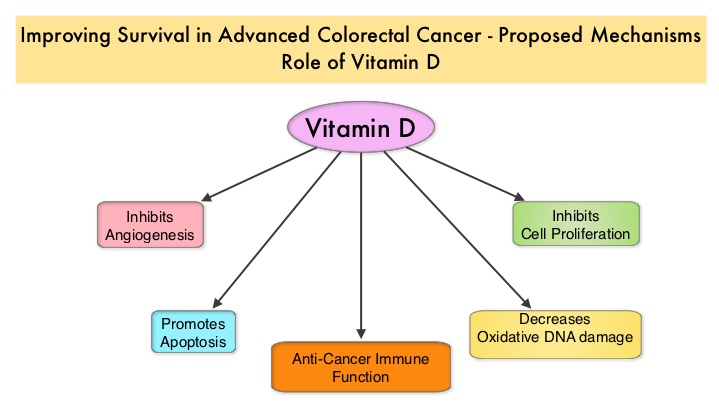 There appears to be a strong association between plasma 25(OH)D level and colorectal cancer (CRC) specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25(OH)D (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). The researchers in this present study conducted a prospective analysis of data from CALGB 80405 trial and evaluated the relationship between plasma 25(OH)D level and patient outcomes, which included Overall Survival and Progression Free Survival (PFS). CALGB 80405 is a phase III trial in which patients with newly diagnosed, advanced colorectal cancer were initially randomized to three groups- 1) Chemotherapy (FOLFIRI or mFOLFOX6) with ERBITUX® (Cetuximab) 2) Chemotherapy with AVASTIN® (Bevacizumab) 3) Chemotherapy with ERBITUX® and AVASTIN®. The protocol was later amended to only include patients with KRAS Wild Type tumors and the chemotherapy with ERBITUX® and AVASTIN® group was deleted. This trial was not designed to compare chemotherapy regimens. The Overall Survival (OS) in both the treatment groups were similar at 29+ months.
There appears to be a strong association between plasma 25(OH)D level and colorectal cancer (CRC) specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25(OH)D (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). The researchers in this present study conducted a prospective analysis of data from CALGB 80405 trial and evaluated the relationship between plasma 25(OH)D level and patient outcomes, which included Overall Survival and Progression Free Survival (PFS). CALGB 80405 is a phase III trial in which patients with newly diagnosed, advanced colorectal cancer were initially randomized to three groups- 1) Chemotherapy (FOLFIRI or mFOLFOX6) with ERBITUX® (Cetuximab) 2) Chemotherapy with AVASTIN® (Bevacizumab) 3) Chemotherapy with ERBITUX® and AVASTIN®. The protocol was later amended to only include patients with KRAS Wild Type tumors and the chemotherapy with ERBITUX® and AVASTIN® group was deleted. This trial was not designed to compare chemotherapy regimens. The Overall Survival (OS) in both the treatment groups were similar at 29+ months.
In the present study, plasma 25(OH)D level were measured at baseline in 1,043 patients at the time of their enrollment in CALGB 80405, and dietary and lifestyle behaviors were collected from self-administered questionnaires. The median plasma 25(OH)D level was 17.2 ng/mL, with the range varying from 2.2 to 72.7 ng/mL (recommended range is 20-30 ng/mL). Factors associated with lower 25(OH)D level included older age, black race, lower dietary and supplemental vitamin D intake, higher Body Mass Index (BMI), ECOG performance status of 1 versus 0 and lower physical activity. Additionally, patients whose blood specimens were drawn in the winter and spring months had significantly lower 25(OH)D level, as did patients who were from the Northern and Northeastern parts of the United States. Vitamin D supplement use was uncommon in this patient population. After adjusting for pathologic and clinical prognostic factors, patients in the group with the highest level of 25(OH)D had significantly improved median OS compared to those in the group with the lowest level (32.6 vs 24.5 months; HR=0.67, P trend 0.002). Higher level of 25(OH)D was also associated with improved PFS (median 12.2 vs 10.1 months; HR 0.80, P trend = 0.02). These results were consistent across all subgroups of patients. The authors concluded that higher plasma level of 25(OH)D was associated with significantly improved survival in metastatic CRC patients treated with a combination of chemotherapy and biologic agents. With 30-35% of the malignancies attributed to dietary habits, the onus is therefore on the treating physicians to provide nutrition counseling during and after cancer treatment. Recommending vitamin D supplements for those patients with colon cancer with low vitamin D levels, may therefore not be unreasonable. Vitamin D status and survival of metastatic colorectal cancer patients: Results from CALGB/SWOG 80405 (Alliance). Ng K, Venook AP, Sato K, et al. J Clin Oncol 33, 2015 (suppl 3; abstr 507)
Long Term Survival with YERVOY® in Advanced Malignant Melanoma
SUMMARY: The American Cancer Society’s estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. The incidence of melanoma has been on the rise for the past 30 years. The US Food and Drug Administration approved YERVOY® (Ipilimumab) for the treatment of unresectable or metastatic melanoma in March 2011. This therapy was the first, to improve Overall Survival in a phase III trial. YERVOY® is a fully human immunoglobulin G1 monoclonal antibody, that blocks Immune checkpoint protein/receptor CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), and this is the first Immune checkpoint protein that was clinically targeted.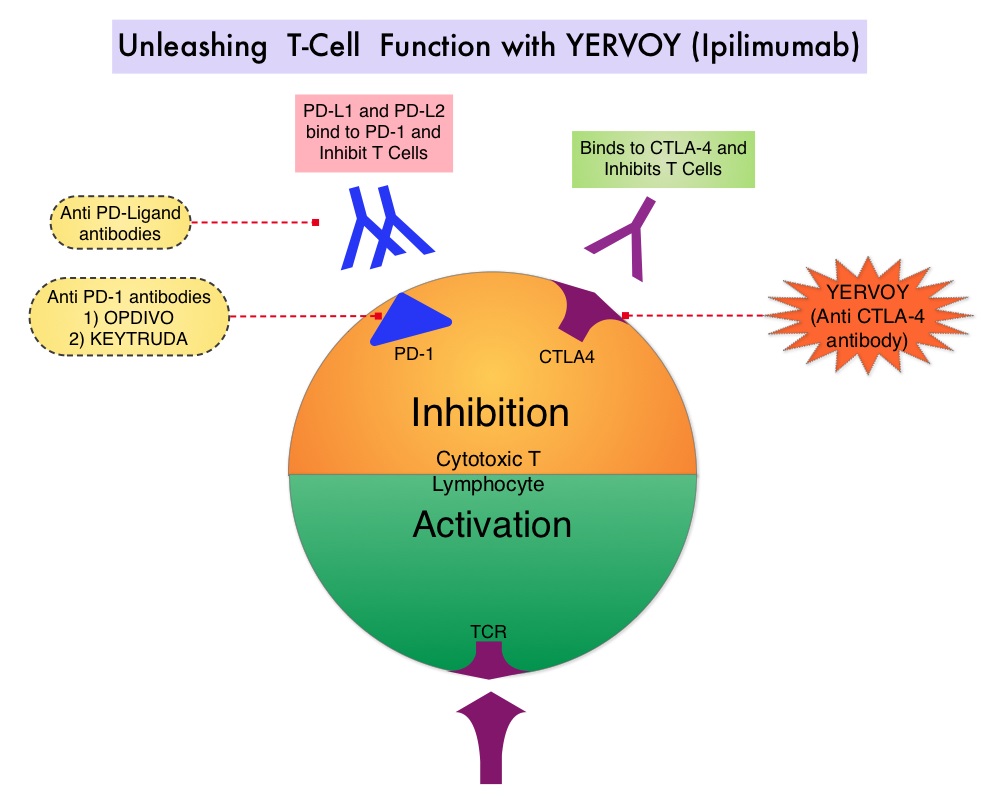 Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4, also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. CA184-024 is a phase III trial in which treatment naïve patients with stage IIIc, N3 (unresectable), or stage IV melanoma were randomly assigned to receive YERVOY® 10 mg/kg IV given along with Dacarbazine 850 mg/m2 IV (N=250) or placebo plus Dacarbazine 850 mg/m2 IV (N=252) administered every 3 weeks for 4 doses followed by Dacarbazine given alone every 3 weeks through week 22. Responders and those with stable disease from week 12 through week 24 were then allowed to receive YERVOY® or placebo as maintenance therapy given every 12 weeks beginning at week 24, until disease progression or unacceptable toxicity. Dacarbazine was not given during the maintenance phase. The median Overall Survival was significantly longer in the group treated with YERVOY® plus Dacarbazine than in patients treated with placebo plus Dacarbazine (11.2 vs 9.1 months, HR=0.72; P< 0.001). This Overall Survival benefit was maintained after 3 years of follow up and toxicities were manageable.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4, also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. CA184-024 is a phase III trial in which treatment naïve patients with stage IIIc, N3 (unresectable), or stage IV melanoma were randomly assigned to receive YERVOY® 10 mg/kg IV given along with Dacarbazine 850 mg/m2 IV (N=250) or placebo plus Dacarbazine 850 mg/m2 IV (N=252) administered every 3 weeks for 4 doses followed by Dacarbazine given alone every 3 weeks through week 22. Responders and those with stable disease from week 12 through week 24 were then allowed to receive YERVOY® or placebo as maintenance therapy given every 12 weeks beginning at week 24, until disease progression or unacceptable toxicity. Dacarbazine was not given during the maintenance phase. The median Overall Survival was significantly longer in the group treated with YERVOY® plus Dacarbazine than in patients treated with placebo plus Dacarbazine (11.2 vs 9.1 months, HR=0.72; P< 0.001). This Overall Survival benefit was maintained after 3 years of follow up and toxicities were manageable.
The authors in this publication conducted a milestone survival analysis with a minimum follow-up of 5 years and they noted that the 5-year survival rate doubled and was 18.2% for patients treated with YERVOY® plus Dacarbazine versus 8.8% for patients treated with placebo plus Dacarbazine (P=0.002). The plateau in the survival curve began at approximately 3 years. In patients who survived at least 5 years and continued maintenance YERVOY®, grade 3 or 4 immune-related adverse events were observed exclusively in the skin. Based on these findings, it was concluded that patients treated with YERVOY® for advanced melanoma, continue to respond following a period of stable disease and these ongoing and durable responses may contribute to long term survival in some patients. Five-Year Survival Rates for Treatment-Naive Patients With Advanced Melanoma Who Received Ipilimumab Plus Dacarbazine in a Phase III Trial. Maio M, Grob J, Aamdal S, et al. J Clin Oncol 2015;33: 1191-1196
Oral Bisphosphonates May Decrease the Risk of Postmenopausal Endometrial Cancer
SUMMARY: Cancer of the endometrium is the most common cancer of the female reproductive organs in the United States. The American Cancer Society estimates that for 2015, about 54,870 new cases of cancer of the body of the uterus will be diagnosed and 10,170 women will die of the disease. Risk factors include age, factors that influence hormone levels such as obesity and estrogen replacement therapy, family history, diet and exercise, drugs such as Tamoxifen, etc.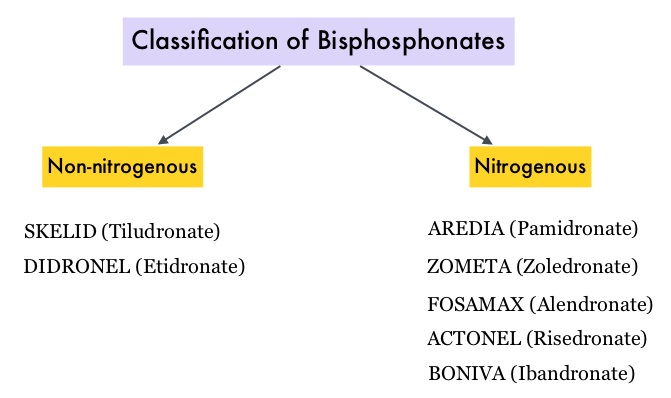 Bisphosphonates such as DIDRONEL® (Etidronate) have been available in the US since the late 1970’s. Bisphosphonates inhibit bone resorption (loss of bone mass) and are indicated for the treatment of osteoporosis and related diseases. The risk of fractures in postmenopausal women with osteoporosis is significantly reduced with the use of bisphosphonates. Several bisphosphonate derivatives have been developed for the treatment of osteoporosis. Aminobisphosphonates such as AREDIA® (Pamidronate) and ZOMETA® (Zoledronic acid) are an integral part of patient management in oncology practice, when bone metastases are initially diagnosed and may have cytostatic, proapoptotic, and antimetastatic properties. An estimated 14.7 million prescriptions for oral bisphosphonates were dispensed in U.S. retail pharmacies in 2012. Previously published studies have shown an inverse relationship between bisphosphonate use and breast cancer risk. Endometrial cancers which are hormone mediated, share risk factors with breast cancer and women with a history of fractures have a lower risk of endometrial cancers.
Bisphosphonates such as DIDRONEL® (Etidronate) have been available in the US since the late 1970’s. Bisphosphonates inhibit bone resorption (loss of bone mass) and are indicated for the treatment of osteoporosis and related diseases. The risk of fractures in postmenopausal women with osteoporosis is significantly reduced with the use of bisphosphonates. Several bisphosphonate derivatives have been developed for the treatment of osteoporosis. Aminobisphosphonates such as AREDIA® (Pamidronate) and ZOMETA® (Zoledronic acid) are an integral part of patient management in oncology practice, when bone metastases are initially diagnosed and may have cytostatic, proapoptotic, and antimetastatic properties. An estimated 14.7 million prescriptions for oral bisphosphonates were dispensed in U.S. retail pharmacies in 2012. Previously published studies have shown an inverse relationship between bisphosphonate use and breast cancer risk. Endometrial cancers which are hormone mediated, share risk factors with breast cancer and women with a history of fractures have a lower risk of endometrial cancers. The association between bisphosphonate use and endometrial cancer has remained unclear except for a few, small retrospective studies, which have shown an inverse relationship. To further explore this observation, the authors conducted a large prospective study which included 89,918 postmenopausal women participating in the Women’s Health Initiative (WHI). Following a health information interview conducted at baseline, the use of bisphosphonate was ascertained at baseline and over the follow up period. All women had an intact uterus at the time of enrollment and the most common type of bisphosphonate used was FOSAMAX® (Alendronate). During the study median follow up of 12.5 years, 1,123 women were diagnosed with incident invasive endometrial cancer. Ever use of bisphosphonates was inversely associated with age-adjusted endometrial cancer risk (HR=0.76; P=0.01). There was no evidence of statistically significant interactions with age at baseline, BMI (Body Mass Index), or hip fracture probability score. In this large prospective cohort of postmenopausal women, the authors concluded that bisphosphonate use was associated with a statistically significant reduction in endometrial cancer risk, in postmenopausal women. Oral Bisphosphonate Use and Risk of Postmenopausal Endometrial Cancer. Newcomb PA, Passarelli MN, Phipps AI, et al. J Clin Oncol 2015; 33: 1186-1190
The association between bisphosphonate use and endometrial cancer has remained unclear except for a few, small retrospective studies, which have shown an inverse relationship. To further explore this observation, the authors conducted a large prospective study which included 89,918 postmenopausal women participating in the Women’s Health Initiative (WHI). Following a health information interview conducted at baseline, the use of bisphosphonate was ascertained at baseline and over the follow up period. All women had an intact uterus at the time of enrollment and the most common type of bisphosphonate used was FOSAMAX® (Alendronate). During the study median follow up of 12.5 years, 1,123 women were diagnosed with incident invasive endometrial cancer. Ever use of bisphosphonates was inversely associated with age-adjusted endometrial cancer risk (HR=0.76; P=0.01). There was no evidence of statistically significant interactions with age at baseline, BMI (Body Mass Index), or hip fracture probability score. In this large prospective cohort of postmenopausal women, the authors concluded that bisphosphonate use was associated with a statistically significant reduction in endometrial cancer risk, in postmenopausal women. Oral Bisphosphonate Use and Risk of Postmenopausal Endometrial Cancer. Newcomb PA, Passarelli MN, Phipps AI, et al. J Clin Oncol 2015; 33: 1186-1190
Post Operative Radiation Therapy (PORT) Improves Survival in Resected N2 Non-Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 221,200 new cases of lung cancer will be diagnosed in the United States in 2015 and over 158,000 patients will die of the disease. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Based on the extent of the disease and the treatment approach, patients with localized or locally advanced NSCLC can be divided into two groups – 1) Surgically resectable disease group (stage I, stage II, and selected stage III tumors) for whom postoperative Cisplatin-based combination chemotherapy may provide a survival advantage (particularly for those with resected stage II or stage IIIA NSCLC). 2) Locally (T3–T4) and/or regionally (N2–N3) advanced disease group who have unresectable disease, who benefit with radiation therapy in combination with chemotherapy. Based on available evidence, postoperative chemotherapy is not recommended outside of a clinical trial for patients with completely resected stage I NSCLC. Although there is sufficient evidence for postoperative chemotherapy in patients with stage II or stage IIIA NSCLC, its usefulness in patients with stage IB NSCLC remains unclear. The value of adjuvant Post Operative Radiation Therapy (PORT) has been evaluated and has not been found to improve the outcome of patients with completely resected stage I NSCLC. The risk of locoregional recurrence (LRR) is 20-40% in patients with resected node-positive disease and this in turn may independently contribute to worsened Overall Survival in patients with NSCLC. Nonetheless, a large meta-analysis from trials conducted mainly in the 1960’s and 1970’s showed that adjuvant Post Operative Radiation Therapy (PORT) in stages IIA NSCLC and IIB NSCLC was associated with an 18% relative increase in the risk of death compared with surgery alone. This decrease in Overall Survival has been attributed to outmoded RT techniques and doses resulting in cardiac and pulmonary toxicity. This study was conducted to evaluate the impact of modern (Computed Tomography simulation and at least Linear accelerator- Linac based, three-dimensional, conformal RT) Post Operative Radiation Therapy (PORT) on Overall Survival (OS) in a large population-based registry of patients with completely resected stage IIIA (N2) NSCLC, when compared with adjuvant chemotherapy alone. The authors identified 4,483 patients in the National Cancer Data Base from 2006 to 2010 with pathologic N2, NSCLC, who underwent complete resection and adjuvant chemotherapy. This large patient population was representative of typical patients treated throughout the United States. Of these large cohort of patients, 1,850 had received PORT (45 Gy or more) and 2,633 patients did not. The authors evaluated the impact of patient and treatment variables on OS. The median follow-up time was 22 months. The use of PORT was associated with an increase in median and 5-year OS compared with no PORT (median OS, 45.2 vs 40.7 months, respectively; 5-year OS, 39.3% vs 34.8% respectively; P= 0.014). The improved OS remained, independently predicted by younger age, female sex, urban population, fewer comorbidities, smaller tumor size, multiagent chemotherapy, resection with at least a lobectomy, and PORT. The authors concluded that modern Post Operative Radiotherapy Therapy confers an additional OS advantage beyond that achieved with adjuvant chemotherapy alone, for patients with N2, NSCLC after complete resection and adjuvant chemotherapy. Postoperative Radiotherapy for Pathologic N2 Non–Small-Cell Lung Cancer Treated With Adjuvant Chemotherapy: A Review of the National Cancer Data Base. Robinson CG, Patel AP, Bradley JD, et al. J Clin Oncol. 2015; 33:870-876
Although there is sufficient evidence for postoperative chemotherapy in patients with stage II or stage IIIA NSCLC, its usefulness in patients with stage IB NSCLC remains unclear. The value of adjuvant Post Operative Radiation Therapy (PORT) has been evaluated and has not been found to improve the outcome of patients with completely resected stage I NSCLC. The risk of locoregional recurrence (LRR) is 20-40% in patients with resected node-positive disease and this in turn may independently contribute to worsened Overall Survival in patients with NSCLC. Nonetheless, a large meta-analysis from trials conducted mainly in the 1960’s and 1970’s showed that adjuvant Post Operative Radiation Therapy (PORT) in stages IIA NSCLC and IIB NSCLC was associated with an 18% relative increase in the risk of death compared with surgery alone. This decrease in Overall Survival has been attributed to outmoded RT techniques and doses resulting in cardiac and pulmonary toxicity. This study was conducted to evaluate the impact of modern (Computed Tomography simulation and at least Linear accelerator- Linac based, three-dimensional, conformal RT) Post Operative Radiation Therapy (PORT) on Overall Survival (OS) in a large population-based registry of patients with completely resected stage IIIA (N2) NSCLC, when compared with adjuvant chemotherapy alone. The authors identified 4,483 patients in the National Cancer Data Base from 2006 to 2010 with pathologic N2, NSCLC, who underwent complete resection and adjuvant chemotherapy. This large patient population was representative of typical patients treated throughout the United States. Of these large cohort of patients, 1,850 had received PORT (45 Gy or more) and 2,633 patients did not. The authors evaluated the impact of patient and treatment variables on OS. The median follow-up time was 22 months. The use of PORT was associated with an increase in median and 5-year OS compared with no PORT (median OS, 45.2 vs 40.7 months, respectively; 5-year OS, 39.3% vs 34.8% respectively; P= 0.014). The improved OS remained, independently predicted by younger age, female sex, urban population, fewer comorbidities, smaller tumor size, multiagent chemotherapy, resection with at least a lobectomy, and PORT. The authors concluded that modern Post Operative Radiotherapy Therapy confers an additional OS advantage beyond that achieved with adjuvant chemotherapy alone, for patients with N2, NSCLC after complete resection and adjuvant chemotherapy. Postoperative Radiotherapy for Pathologic N2 Non–Small-Cell Lung Cancer Treated With Adjuvant Chemotherapy: A Review of the National Cancer Data Base. Robinson CG, Patel AP, Bradley JD, et al. J Clin Oncol. 2015; 33:870-876
Intraperitoneal Chemotherapy Superior to IV Chemotherapy in Advanced Ovarian Cancer
SUMMARY: The American Cancer Society estimates that over 21,000 women will be diagnosed with ovarian cancer in the United States for 2015 and over 14,000 will die of the disease. Ovarian cancer ranks fifth in cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system. Intraperitoneal (IP) delivery of antineoplatic drugs ("Belly Bath") for ovarian cancer dates back to the late 1970’s and 1980’s. This strategy for ovarian cancer was based on the fact that the peritoneal cavity is the primary site of spread and failure in most cases of advanced ovarian cancer. 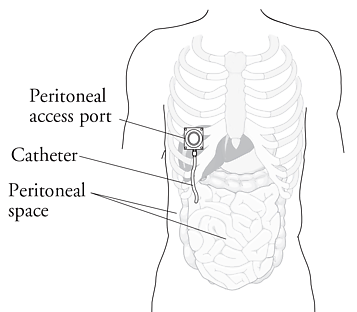 IP chemotherapy for ovarian cancer facilitates the exposure of tumors in the peritoneal cavity to 10-20 fold greater concentration of Cisplatin and Carboplatin and 1000 fold greater concentration of Paclitaxel, compared to IV administration, thus allowing continuous and prolonged exposure of the tumor to high drug concentrations, without systemic toxicities. Even though three Intergroup Phase III trials demonstrated the superiority of IP therapy over IV therapy, it has not been widely accepted in the US and abroad. Barriers to IP therapy have included inconvenience, IP catheter related complications, higher toxicities, lack of knowledge regarding patient selection for IP therapy as well as minimum number of cycles of IP therapy to administer and uncertain long term benefit.
IP chemotherapy for ovarian cancer facilitates the exposure of tumors in the peritoneal cavity to 10-20 fold greater concentration of Cisplatin and Carboplatin and 1000 fold greater concentration of Paclitaxel, compared to IV administration, thus allowing continuous and prolonged exposure of the tumor to high drug concentrations, without systemic toxicities. Even though three Intergroup Phase III trials demonstrated the superiority of IP therapy over IV therapy, it has not been widely accepted in the US and abroad. Barriers to IP therapy have included inconvenience, IP catheter related complications, higher toxicities, lack of knowledge regarding patient selection for IP therapy as well as minimum number of cycles of IP therapy to administer and uncertain long term benefit.
The authors in this study retrospectively analyzed data from 876 patients in the two phase III, Gynecologic Oncology Group trials (GOG#114 and GOG#172). The purpose of this study was to determine the long-term survival and associated prognostic factors following IP chemotherapy, in patients with advanced ovarian cancer. In both studies, patients were randomly assigned to IP (combined N=440) or IV (combined N=436) chemotherapy. In GOG#114 trial, the two treatment groups were Paclitaxel at 135 mg/m2 IV followed by Cisplatin 75 mg/m2 IV for 6 cycles or Carboplatin IV for 2 courses followed by Paclitaxel 135 mg/m2 IV dose on day 1 and Cisplatin 100 mg/m2 IP on day 8, for 6 cycles. In GOG#172 trial, the two treatment groups (IV vs IP) were Paclitaxel at 135 mg/m2 IV followed by Cisplatin 75 mg/m2 IV on day 2 for 6 cycles or Cisplatin 100mg/m2 IP on day 2 and Paclitaxel 60 mg/m2 IP on day 8, for 6 cycles. Patients in the IP and IV groups were well balanced for baseline characteristics. At a median follow up of 10.7 years, the median Overall Survival with IP chemotherapy was 61.8 months compared with 51.4 months for IV chemotherapy and IP chemotherapy resulted in a 23% reduction in the risk of death (HR=0.77; P=0.002). IP chemotherapy was also associated with improved survival among those patients with gross residual disease ie.1 cm or less (HR = 0.75; P=0.006). The risk for death decreased by 12% for each cycle of IP chemotherapy that patients completed (HR=0.88; P<0.001). Factors significantly associated with poorer Overall Survival included clear/mucinous vs serous histology (HR=2.79; P <0 .001), gross residual vs no visible disease (HR=1.89; P< 0.001), and fewer vs more cycles of IP chemotherapy (HR=0.88; P<0.001). Younger patients were more likely to complete IP chemotherapy, with probability of completion decreasing by 5% with each additional year of age (P<0.001). The authors concluded that IP chemotherapy was associated with significantly prolonged Overall Survival in women with advanced ovarian cancer, including those with gross residual disease, when compared with IV chemotherapy. This benefit extends beyond 10 years and Overall Survival improved with increasing number of IP chemotherapy cycles administered. Long-Term Survival Advantage and Prognostic Factors Associated With Intraperitoneal Chemotherapy Treatment in Advanced Ovarian Cancer: A Gynecologic Oncology Group Study . Tewari D, Java J, Salani R, et al. JCO published online on March 23, 2015; DOI:10.1200/JCO.2014.55.9898.
Clinical Cancer Advances 2015 Annual Report on Progress Against Cancer From the American Society of Clinical Oncology (PART II)
SUMMARY: Cancer mortality rates in the United States have declined 20% from their peak of 215 per 100,000 in 1991 to 172 per 100,000 in 2010. Part II of this Annual Report on Progress Against Cancer explores, ADVANCES IN TREATMENT, ADVANCES IN TUMOR BIOLOGY AND ADVANCES IN PATIENT CARE. Clinical study details for several of these studies can be accessed at www.oncoprescribe.com
ADVANCES IN TREATMENT
COMBINATION THERAPY
Chemotherapy and Radiotherapy significantly improves Survival for Patients with Low-Grade Glioma – Radiotherapy has been the standard first-line treatment for patients with low-grade glioma. In a study involving 251 patients with gliomas which included grade 2 Astrocytoma, Oligoastrocytoma, or Oligodendroglioma, the addition of chemotherapy (PCV regimen – (Procarbazine, Lomustine, and Vincristine) to radiation extended median survival by 5.5 years (13.3 vs 7.8 years, P=0.03; HR=0.59) and also resulted in a longer median Progression Free survival (10.4 vs 4 years, P=0.002; HR=0.50), when compared with radiotherapy alone.
First-Line Chemotherapy Added to Hormone Therapy Improves Survival for Men With Advanced Prostate Cancer – Androgen Deprivation Therapy (ADT) has been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy. In a pivotal phase III study which included 790 men with metastatic, hormone-sensitive prostate cancer, the addition of TAXOTERE® (Docetaxel) chemotherapy to ADT improved median Overall Survival by 10 months from 42.3 months in the ADT alone group to 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001).
TARGETED THERAPY
Overcoming Resistance to EGFR Targeted Agents in Lung Cancer– TARCEVA® (Erlotinib) and GILOTRIF® (Afatinib) are recommended as first-line treatments for patients harboring Epidermal Growth Factor Receptor (EGFR) gene mutations, which are detected in 15% of Caucasian and 40% of Asian patients with NSCLC. An additional EGFR mutation (T790M) is responsible for resistance to EGFR-targeted therapy and may be detected in about 50% of those harboring EGFR mutations in Non Small Cell Lung Cancer (NSCLC). AZD9291 and CO-1686 are two new agents which have demonstrated a 50-60% response rate in patients with T790M mutation.
New second-line treatment options for ALK-positive NSCLC – XALKORI® (Crizotinib) significantly improves PFS and Response Rates in patients with ALK-positive NSCLC. Approximately one third of these patients are resistant to XALKORI® and this has been attributed to acquired mutation within the ALK tyrosine kinase domain, amplification of the ALK fusion gene, subtherapeutic inhibition of ALK tyrosine kinase or activation of other pathways that can cause abnormal cell proliferation. ZYKADIA® (Ceritinib) resulted in an overall Response Rate (RR) of 58% and median PFS of 7 months in this patient population, with responses seen in untreated CNS lesions as well.
FDA Approves First Treatment for Chemotherapy-Resistant, Advanced Stomach Cancer – CYRAMZA® (Ramucirumab) an angiogenesis inhibitor was approved for the treatment of advanced stomach cancer or gastroesophageal junction adenocarcinoma that progressed during or after chemotherapy. The approval was based on a phase III trial involving 355 patients in which patients treated with CYRAMZA® (Ramucirumab) had longer survival (5.2 vs 3.8 months, HR = 0.78, P=0.047), when compared to placebo.
Lenvatinib: A New Option for a Difficult-to-Treat Thyroid Cancer – LENVIMA® (Lenvatinib) a Tyrosine Kinase Inhibitor, was approved for the treatment of patients with locally recurrent or metastatic, progressive, RadioActive Iodine (RAI)-refractory Differentiated Thyroid Cancer (DTC). In a phase III study involving 392 patients with advanced RAI-refractory Differentiated Thyroid Cancer (DTC), the median Progression Free Survival was 18.3 months in the LENVIMA® group and 3.6 months in the placebo group (HR= 0.21; P<0.001). The objective response rate with LENVIMA® was 64.8% versus 1.5% with placebo (P<0.001). Another targeted drug, NEXAVAR® (Sorafenib), was approved for the same patient population in 2013.
IMMUNOTHERAPY
Both antibody and cell-based immunotherapy approaches have taken center stage in cancer immunotherapy. Check point inhibitors such as anti CTLA-4. Anti PD-1 and anti PDL1 antibodies unleash the immune system allowing the T cells to attack the malignant cells.
Adjuvant Immunotherapy For Early Stage Melanoma – YERVOY® (Ipilimumab), an anti CTLA-4 antibody decreased the relative risk of recurrence by 25% (HR=0.75; P=0.0013), when compared with placebo, in patients with high risk, completely resected, stage III melanoma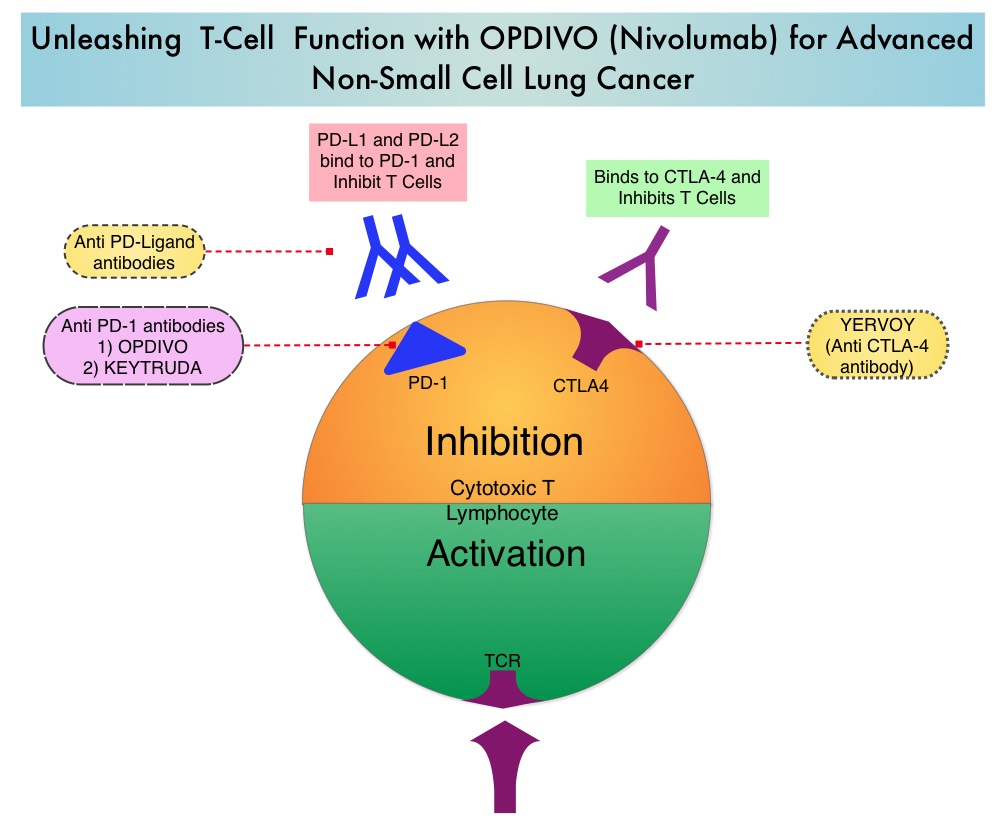
Immunotherapy in Lung Cancer – OPDIVO® (Nivolumab), a PD-1 targeted monoclonal antibody improved overall survival by 41% (HR=0.59; P=0.00025) when compared to TAXOTERE® (Docetaxel) in a randomized phase III trial in patients with metastatic squamous NSCLC, who had experienced disease progression during or after one prior platinum-based chemotherapy regimen. KEYTRUDA® (Pembrolizumab) another PD-1 antibody demonstrated superior responses in patients with lung cancer when the tumors were PD-L1 positive versus PD-L1 negative (23% vs 9%) suggesting that PD-L1 may be a predictor of response to PD-1 and PD-L1 therapies.
Tumor Directed Chimeric Antigen Receptor (CAR) T-Cell Therapy – In this type of immunotherapy, T cells are collected from the patient’s own blood and are genetically engineered to produce special receptors on their surface called chimeric antigen receptors (CAR’s). These cytotoxic T cells with these chimeric antigen receptors on their surface are now able to recognize a specific antigen on tumor cells. These engineered CAR T-cells (CTL019) which are grown in vitro, are then infused into the patient and they in turn proliferate in the patient’s body, recognize and kill cancer cells expressing that specific antigen. In a small study of patients with relapsed or refractory ALL, treatment with autologous Chimeric Antigen Receptor (CAR) T-cells (CTL019 T-cells) resulted in a 90% remission rate with sustained remissions for up to 2 years and overall survival of 78%.
ADVANCES IN TUMOR BIOLOGY
Genomic Profile Based Therapy – In a recent landmark study, analysis of molecular data from approximately 3,500 patients with 12 different forms of cancer, lead to the identification of 11 major molecular subtypes. It was noted that most malignancies that originated from the same tissue or organ had similar genomic profiles. Treatment in the foreseeable future may be based on genomic profile rather than site of origin of the malignancy.
Blood Test Predicts Resistance to Prostate Cancer Therapy – In patients with metastatic CRPC, the presence of Androgen Receptor Variant AR-V7 rather than a normal androgen receptor, in the circulating tumor cells (CTCs) before, during, and after treatment with either XTANDI® (Enzalutamide) or ZYTIGA® (Abiraterone), conferred resistance to these agents. The Androgen Receptor Variant AR-V7 was detected in roughly 40% of patients treated with XTANDI® and 20% of those treated with ZYTIGA®.
Gut Bacteria and Response to Therapy – Two early studies have demonstrated that Intestinal bacteria may be beneficial in priming and mobilizing immune cells to attack tumors. Further, certain chemotherapeutic agents such as CYTOXAN® (Cyclophosphamide) and ELOXATIN® (Oxaliplatin) were less effective when intestinal bacteria were eradicated with antibiotics.
ADVANCES IN PATIENT CARE
Early Initiation of Palliative Care Improves Patient Well Being – In a study of close to 500 patients with advanced cancer early palliative care improved, quality of life at the end of life, spiritual well-being, symptom severity, and satisfaction with care at 4 months after diagnosis.
Pregnancy After Breast Cancer Treatment – Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. Two studies reported a promising new way of preserve fertility. In the POEMS (Prevention of Early Menopause Study) phase III trial, the addition of ZOLADEX® (Goserelin) to chemotherapy decreased the POF to 8% compared to 22% with chemotherapy alone (P=0.04). More women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). In a different study, similar findings were noted with the addition of another hormonal agent, TRELSTAR® (Triptorelin) to chemotherapy.
Masters GA, Krilov L, Bailey HH , et al. Published online before print January 20, 2015, doi: 10.1200/JCO.2014.59.9746
Cancers associated with BRCA1 and BRCA2 mutations other than Breast and Ovarian
SUMMARY:Approximately 5-10% of all Breast and Ovarian cancers are caused by inherited genetic factors and are typically the result from inherited mutations in either the BRCA1 or BRCA2 gene. BRCA1 and BRCA2 are tumor suppressor genes located on chromosome 17 and chromosome 13 respectively.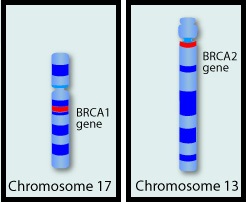 They control cell growth by repairing DNA damage and thus prevent tumor development. Mutations in these genes predispose an individual to develop malignant tumors. The presence of BRCA1 and BRCA2 mutations can significantly increase the lifetime risk for developing Breast and Ovarian cancer, as high as 85% and 40% respectively. While the association of BRCA1 and BRCA2 mutations with Breast and Ovarian cancer risks is well-established, the potential association of these mutations with other cancers has remained unclear. This study was conducted to evaluate the incidence of cancers other than Breast and Ovarian cancer, in known BRCA1 and BRCA2 mutation carriers. The study population included 1072 patients who had received genetic counseling at the UT MD Anderson Cancer Center between 1997 and 2013 and had a confirmed BRCA1 (N=613) or BRCA2 (N=459) mutation. The authors then compared the cancer incidence of the study population with the United States Cancer Statistics. The expected and observed numbers of cancer cases were calculated at 5 year intervals to take into consideration different age-related incidence rates. Standardized Incidence Ratios (SIRs) for each cancer type was then calculated for the entire sample and for BRCA1 mutation carriers and BRCA2 mutation carriers separately. {Standardized Incidence Ratio (SIR) is used to determine if the occurrence of cancer in a relatively small population is high or low. A SIR of 1 would indicate no increase or decrease, SIR of 1.5 indicates an excess of 50%, SIR of 2 indicates an excess of 100%}. Among the study population, 1177 cancers comprising 30 different cancer types were identified. There was no significant increase in cancers other than Breast and Ovarian cancer in individuals with BRCA1 mutation, but there was a trend of increasing incidence of Melanoma in BRCA1 mutation carriers, compared to general population. Individuals with BRCA2 mutation however had significantly higher incidence of Pancreatic cancer in both men and women (SIR= 21.7; P < 0.001), Prostate cancer in men (SIR= 4.9; P = 0.002) and a trend of increasing incidence of Cervical cancer, compared to general population. The authors concluded that their findings support the NCCN practice guidelines for this patient population which includes screening for male Breast cancer, Prostate cancer and Melanoma, acknowledging that specific screening guidelines for Pancreatic cancer do not exist. Mersch J, Jackson MA, Park M, et al. Cancer 2015; 121:269-275
They control cell growth by repairing DNA damage and thus prevent tumor development. Mutations in these genes predispose an individual to develop malignant tumors. The presence of BRCA1 and BRCA2 mutations can significantly increase the lifetime risk for developing Breast and Ovarian cancer, as high as 85% and 40% respectively. While the association of BRCA1 and BRCA2 mutations with Breast and Ovarian cancer risks is well-established, the potential association of these mutations with other cancers has remained unclear. This study was conducted to evaluate the incidence of cancers other than Breast and Ovarian cancer, in known BRCA1 and BRCA2 mutation carriers. The study population included 1072 patients who had received genetic counseling at the UT MD Anderson Cancer Center between 1997 and 2013 and had a confirmed BRCA1 (N=613) or BRCA2 (N=459) mutation. The authors then compared the cancer incidence of the study population with the United States Cancer Statistics. The expected and observed numbers of cancer cases were calculated at 5 year intervals to take into consideration different age-related incidence rates. Standardized Incidence Ratios (SIRs) for each cancer type was then calculated for the entire sample and for BRCA1 mutation carriers and BRCA2 mutation carriers separately. {Standardized Incidence Ratio (SIR) is used to determine if the occurrence of cancer in a relatively small population is high or low. A SIR of 1 would indicate no increase or decrease, SIR of 1.5 indicates an excess of 50%, SIR of 2 indicates an excess of 100%}. Among the study population, 1177 cancers comprising 30 different cancer types were identified. There was no significant increase in cancers other than Breast and Ovarian cancer in individuals with BRCA1 mutation, but there was a trend of increasing incidence of Melanoma in BRCA1 mutation carriers, compared to general population. Individuals with BRCA2 mutation however had significantly higher incidence of Pancreatic cancer in both men and women (SIR= 21.7; P < 0.001), Prostate cancer in men (SIR= 4.9; P = 0.002) and a trend of increasing incidence of Cervical cancer, compared to general population. The authors concluded that their findings support the NCCN practice guidelines for this patient population which includes screening for male Breast cancer, Prostate cancer and Melanoma, acknowledging that specific screening guidelines for Pancreatic cancer do not exist. Mersch J, Jackson MA, Park M, et al. Cancer 2015; 121:269-275
