SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 231,840 new cases of invasive breast cancer will be diagnosed in 2015 and over 40,000 women will die of the disease. The National Surgical Adjuvant Breast and Bowel Project (NASBP) protocols B-04 and B-06 have clearly established, after more than a 2 decades of evaluation and follow up that, in Stage I and II breast cancer, there is no significant difference in either distant Disease Free or Overall Survival between the breast conserving surgery and breast removal surgery (Mastectomy). This data established breast-sparing surgery as the preferred local-regional procedure. Radiotherapy after breast-conserving surgery significantly decreases the risk of local recurrence and improves breast cancer–specific survival in certain subgroups of patients. Under ideal circumstances, approximately 70% of the patients can be treated with breast conserving surgery and the risk factors for local recurrences include younger age, high tumor grade, negative hormone receptor status, lymphovascular invasion, extensive intraductal component and positive surgical margins. According to the American Cancer Society, 42% of all invasive breast cancers in the US occur in women 65 years of age or older. These patients may have associated comorbidities and may therefore be appropriate candidates for breast-sparing surgery (rather than mastectomy), as post-op recovery time is shorter. Elderly patients often present with breast tumors that are hormone receptor positive and indolent. Whether women 70 years of age or older with early stage breast cancer need radiotherapy after breast conserving surgery, was addressed by the CALGB cooperative group ( CALGB 9343) and it was noted that the addition of radiation did not improve Overall Survival or distant Disease Free Survival, although there was some improvement in locoregional recurrence rate.
The authors in PRIME II study evaluated local control outcomes in elderly women with early stage breast cancer, at low risk of local recurrence at 5 years, by avoiding whole-breast radiotherapy, following breast conserving surgery. In this Phase III randomized trial, 1326 patients 65 years or older, with low risk early breast cancer, who had undergone breast conserving surgery and were receiving adjuvant hormonal treatment, were randomly assigned to receive whole-breast irradiation (N=658) or no further therapy (N=668). Low risk was defined as tumors T1–T2 up to 3 cm in greatest dimension, clear surgical margins, negative axillary nodes and positive hormone receptors. Either lymphovascular invasion or grade 3 tumor histology was permitted, but not both. The primary endpoint was ipsilateral breast tumor recurrence. After median follow up of 5 years, ipsilateral breast tumor recurrence was 1.3% in women assigned to whole-breast radiotherapy and 4.1% in those assigned no radiotherapy (P=0•0002). There was however no differences in regional recurrence, contralateral breast cancers, distant metastases or new breast cancers, noted between the two groups. The 5-year Overall Survival was similar in both groups 93•9% (P=0•34). The authors concluded that the 5-year ipsilateral breast tumor recurrence is probably low enough that radiotherapy could be avoided in a subset of elderly patients with low risk breast cancer following breast conserving surgery. Kunkler IH, Williams LJ, Jack WJ, et al: Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet -Oncol. 2015;16:266-273

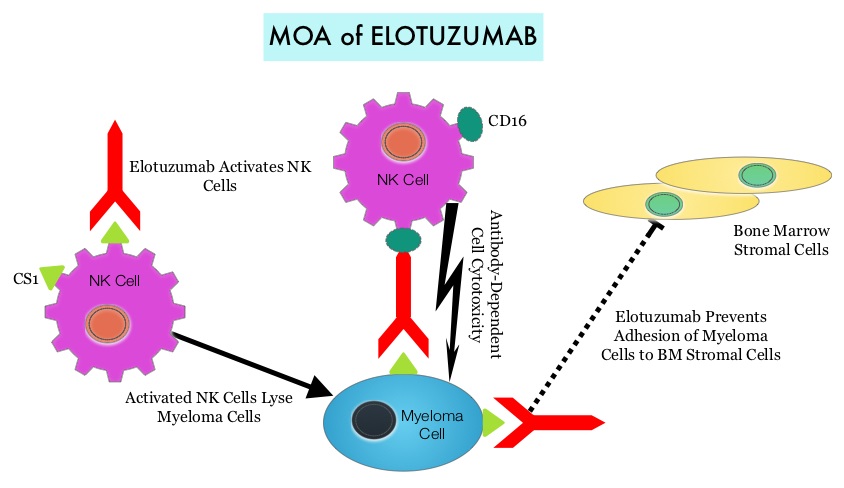 By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s
By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. Previously published phase Ib/II study, has shown encouraging activity, when Elotuzumab was combined with REVLIMID® and Dexamethasone, in patients with Relapsed/Refractory Multiple Myeloma (RRMM). ELOQUENT-2 is an open-label phase III trial in which 646 patients with Relapsed/Refractory Multiple Myeloma were randomized in a 1:1 ratio to receive Elotuzumab in combination with REVLIMID® and Dexamethasone (N=321) or REVLIMID® and Dexamethasone alone (N=325). Enrolled patients had 1–3 prior therapies and were not REVLIMID® refractory. Prior therapies included VELCADE® (Bortezomib), THALOMID® (Thalidomide) and REVLIMID®. Approximately 35% of the enrollees were refractory to the last therapy, 32% had del(17p) and 9% had t(4;14). The median age was 66 years. Elotuzumab was administered at 10 mg/kg IV weekly for the first two cycles and then once every 2 weeks thereafter. REVLIMID® was given at 25 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly. In the Elotuzumab group, Dexamethasone was dosed at 28 mg orally plus 8 mg IV on the weeks when Elotuzumab was administered. The cycle duration was 28 days. Treatment was administered until disease progression or unacceptable toxicity. Primary endpoints were Progression Free Survival (PFS) and Overall Response Rate (ORR). At a median follow up of 24 months, PFS in the Elotuzumab group was 19.4 months compared to 14.9 months in the REVLIMID®/Dexamethasone alone group (HR=0.70; P=0.0004). The 1-year PFS for the Elotuzumab versus control group was 68% vs 57% respectively and the 2-year PFS was 41% vs 27%. This benefit was seen across all subgroups including those with unfavorable cytogenetics. The ORR was 79% in the Elotuzumab group and 66% in the control group. (P = 0.0002). At the time of this interim analysis, more patients in the Elotuzumab group remained on therapy (35%) compared to the control group (21%) and treatment discontinuation was mainly for disease progression. Grade 3–4 toxicities occurred in 15% or more patients in the Elotuzumab group and included neutropenia and anemia. The authors concluded that Elotuzumab with its novel immunotherapeutic mechanism of action, when added to REVLIMID® and Dexamethasone, reduced the risk of disease progression by 30% in patients with Relapsed/Refractory MultipleMyeloma, and this was accomplished with manageable toxicities. Patients in this study are being followed up for long term outcomes including Overall Survival. Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: A phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2015;(suppl; abstr 8508).</s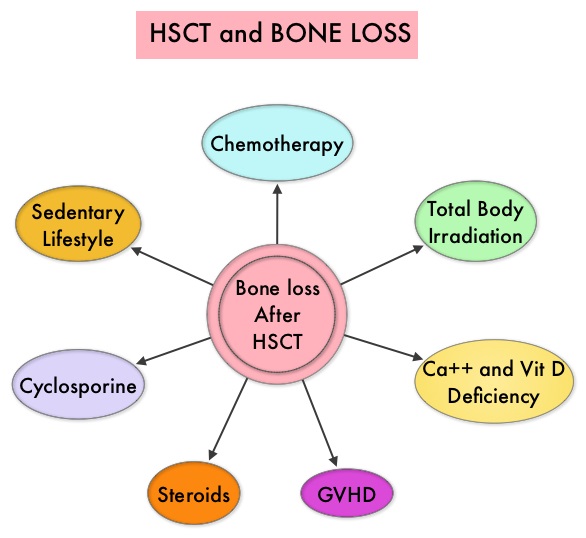 Morbidity and mortality as well as quality of life related to bone loss among long term survivors after HSCT, has been previously published. Factors contributing to bone loss in this patient population include intensive chemotherapy, total body irradiation, post-transplantation glucocorticoid use, reduced intake and metabolism of Calcium and Vitamin D, graft versus host disease, use of Cyclosporine-A and sedentary lifestyle after transplantation. Loss of Bone Mineral Density (BMD) occurs within 6 to 12 months after transplantation at all skeletal sites, followed by initial recovery of BMD in the lumbar spine and a slower recovery of BMD in the femur neck. This bone loss may persist for 48 to 120 months or even longer. The authors in this single institution, retrospective study, calculated the cumulative incidence rates of fractures among survivors of Autologous and Allogeneic Hematopoietic Stem Cell Transplantations (HSCT) and compared the rate of fractures to that of the general US population. Data was collected from 7,620 patients over 18 years of age who underwent HSCT at The University of Texas MD Anderson Cancer Center, from January 1997 to December 2011 and these patients were observed through December 2013. The authors then calculated the cumulative incidence of fractures, with death as a competing risk and the age and sex-specific fracture incidence rates were compared with those in the US general population, using estimated rates from the 1994 National Health Interview Survey and the 2004 National Hospital Discharge Survey. Of the 7,620 patients who underwent HSCT, 56% were male and 51% underwent Autologous and 49% underwent Allogeneic stem cell transplantation.
Morbidity and mortality as well as quality of life related to bone loss among long term survivors after HSCT, has been previously published. Factors contributing to bone loss in this patient population include intensive chemotherapy, total body irradiation, post-transplantation glucocorticoid use, reduced intake and metabolism of Calcium and Vitamin D, graft versus host disease, use of Cyclosporine-A and sedentary lifestyle after transplantation. Loss of Bone Mineral Density (BMD) occurs within 6 to 12 months after transplantation at all skeletal sites, followed by initial recovery of BMD in the lumbar spine and a slower recovery of BMD in the femur neck. This bone loss may persist for 48 to 120 months or even longer. The authors in this single institution, retrospective study, calculated the cumulative incidence rates of fractures among survivors of Autologous and Allogeneic Hematopoietic Stem Cell Transplantations (HSCT) and compared the rate of fractures to that of the general US population. Data was collected from 7,620 patients over 18 years of age who underwent HSCT at The University of Texas MD Anderson Cancer Center, from January 1997 to December 2011 and these patients were observed through December 2013. The authors then calculated the cumulative incidence of fractures, with death as a competing risk and the age and sex-specific fracture incidence rates were compared with those in the US general population, using estimated rates from the 1994 National Health Interview Survey and the 2004 National Hospital Discharge Survey. Of the 7,620 patients who underwent HSCT, 56% were male and 51% underwent Autologous and 49% underwent Allogeneic stem cell transplantation. 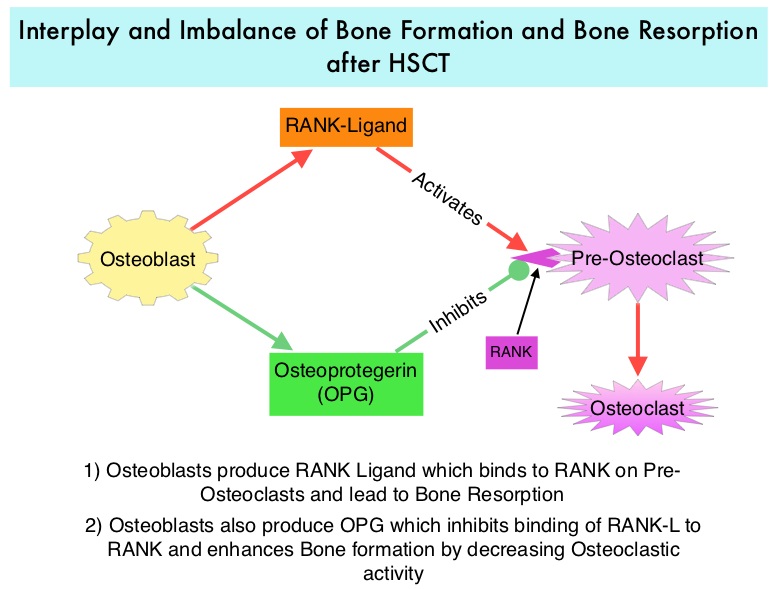 The most common reasons for HSCT were hematologic malignancies other than Multiple Myeloma (67%), Multiple Myeloma (22%) and other solid tumors (11%). The median follow up was 85 months. Fractures occurred in 8% of patients (N = 602) of whom 419 patients had an Autologous stem cell transplantation and 183 patients had Allogeneic stem cell transplantation. The incidence of fracture was higher in patients older than age 50 years, 5 times higher among patients with Multiple Myeloma compared to other hematologic malignancies and patients who underwent Autologous transplantation were 45% more likely to develop a fracture than those who underwent an Allogeneic transplantation. When age- and sex-specific fracture incidence rates after HSCT were compared with National Health Interview Survey data, females were at approximately 8 times greater risk and men 45-64 years old were at approximately 7-9 times greater risk of sustaining a fracture. The authors concluded that the incidence of fractures after HSCT is significantly higher and all patients undergoing HSCT should be considered to be at risk for post-transplantation bone loss. Measures to prevent bone loss and fractures include physical exercise, Vitamin D and Calcium supplementation, avoiding tobacco products, abstaining from excess alcohol intake and fall prevention. The authors recommend that patients undergoing HSCT should have a Dual Energy X-ray Absorptiometry scan performed at baseline and at 6 months following transplantation. Increased Incidence of Fractures in Recipients of Hematopoietic Stem-Cell Transplantation. Pundole XN, Barbo AG, Lin H, et al. J Clin Oncol 2015; 33:1364-1370
The most common reasons for HSCT were hematologic malignancies other than Multiple Myeloma (67%), Multiple Myeloma (22%) and other solid tumors (11%). The median follow up was 85 months. Fractures occurred in 8% of patients (N = 602) of whom 419 patients had an Autologous stem cell transplantation and 183 patients had Allogeneic stem cell transplantation. The incidence of fracture was higher in patients older than age 50 years, 5 times higher among patients with Multiple Myeloma compared to other hematologic malignancies and patients who underwent Autologous transplantation were 45% more likely to develop a fracture than those who underwent an Allogeneic transplantation. When age- and sex-specific fracture incidence rates after HSCT were compared with National Health Interview Survey data, females were at approximately 8 times greater risk and men 45-64 years old were at approximately 7-9 times greater risk of sustaining a fracture. The authors concluded that the incidence of fractures after HSCT is significantly higher and all patients undergoing HSCT should be considered to be at risk for post-transplantation bone loss. Measures to prevent bone loss and fractures include physical exercise, Vitamin D and Calcium supplementation, avoiding tobacco products, abstaining from excess alcohol intake and fall prevention. The authors recommend that patients undergoing HSCT should have a Dual Energy X-ray Absorptiometry scan performed at baseline and at 6 months following transplantation. Increased Incidence of Fractures in Recipients of Hematopoietic Stem-Cell Transplantation. Pundole XN, Barbo AG, Lin H, et al. J Clin Oncol 2015; 33:1364-1370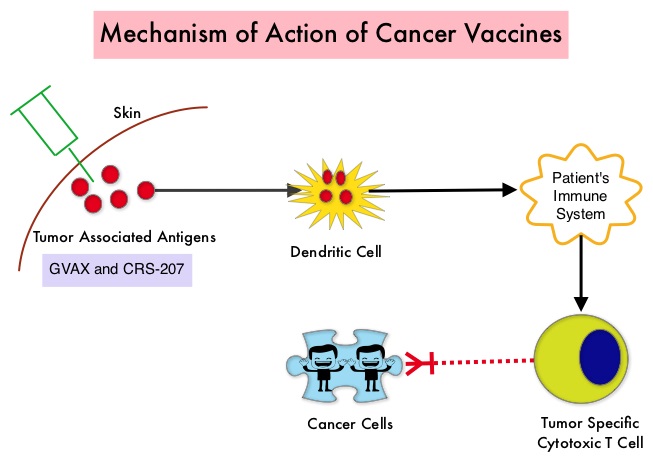 The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333
The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from Pancreatic Cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on Pancreatic Cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic Pancreatic Adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 in Group A and six doses of GVAX alone in the Group B. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The Primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median Overall Survival (OS) was 6.1 months in Group A patients who had received the combination of two vaccines compared to 3.9 months in Group B patients who received GVAX alone (HR=0.59, P=0.02), resulting in a 41% reduction in risk of death with the combination immunotherapy. The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group (Group A) compared with 12% for the GVAX alone group (Group B). The median OS in an updated analysis of patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for those who received three doses of GVAX alone (HR=0.53, P=0.02), a 47% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median OS of 5.7 months compared to 3.7 months with GVAX alone (HR=0.30, P<0.001), a 70% reduction in risk of death. Stabilization or reduction of tumor marker CA 19-9, was seen in 27% of patients receiving combination immunotherapy compared to 9% in those who received GVAX alone (P=0.08). The median OS in patients with stable or better CA 19-9 response was 10.3 months compared with 4 months in those with CA 19-9 progression (HR=0.43, P=0.02). Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved Overall Survival with minimal toxicity, for patients with metastatic Pancreatic Carcinoma, who had failed prior therapies. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 2015; 33:1325-1333
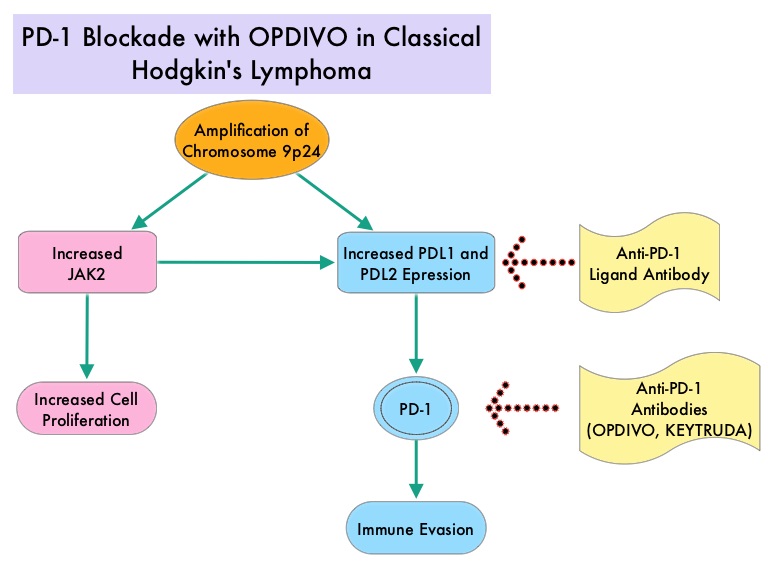 Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-Ls. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin's lymphomas. It would therefore seem logical to block/inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.
Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-Ls. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin's lymphomas. It would therefore seem logical to block/inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.  The authors in this study enrolled 23 patients with relapsed or refractory Hodgkin's lymphoma of whom 87% had received three or more prior therapies. Seventy eight percent (78%) of the patients had previously received ADCETRIS® (Brentuximab) and 78% had undergone autologous stem-cell transplantation. All patients with the exception of one patient had the nodular-sclerosis subtype of Hodgkin's lymphoma. The median age was 35 years. Patients received OPDIVO® (Nivolumab) at a dose of 3 mg/kg every 2 weeks and treatment was continued until patients had a complete response, tumor progression, or severe toxicities. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody. The median number of OPDIVO® doses that patients received was 16 and the median duration of treatment was 36 weeks. The authors noted an Objective Response Rate of 87% with a complete response of 17%, and a partial response of 70%, in this heavily pretreated group of patients. Stable disease was noted in 13% of the patients. The Progression Free Survival at 24 weeks was 86%.Most of the adverse events were grade 1 and grade 2. Analyses of pretreatment tumor specimens revealed increased expression of PDL1 and PDL2. Reed–Sternberg cells showed nuclear positivity of phosphorylated STAT3, suggestive of active JAK-STAT signaling. The authors concluded that OPDIVO® is highly active with favorable toxicities in heavily pretreated relapsed or refractory Hodgkin's lymphoma “highlighting the genetically defined sensitivity to PD-1 blockade”. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. Ansell SM, Lesokhin AM, Borrello I, et al. N Engl J Med 2015; 372:311-319
The authors in this study enrolled 23 patients with relapsed or refractory Hodgkin's lymphoma of whom 87% had received three or more prior therapies. Seventy eight percent (78%) of the patients had previously received ADCETRIS® (Brentuximab) and 78% had undergone autologous stem-cell transplantation. All patients with the exception of one patient had the nodular-sclerosis subtype of Hodgkin's lymphoma. The median age was 35 years. Patients received OPDIVO® (Nivolumab) at a dose of 3 mg/kg every 2 weeks and treatment was continued until patients had a complete response, tumor progression, or severe toxicities. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody. The median number of OPDIVO® doses that patients received was 16 and the median duration of treatment was 36 weeks. The authors noted an Objective Response Rate of 87% with a complete response of 17%, and a partial response of 70%, in this heavily pretreated group of patients. Stable disease was noted in 13% of the patients. The Progression Free Survival at 24 weeks was 86%.Most of the adverse events were grade 1 and grade 2. Analyses of pretreatment tumor specimens revealed increased expression of PDL1 and PDL2. Reed–Sternberg cells showed nuclear positivity of phosphorylated STAT3, suggestive of active JAK-STAT signaling. The authors concluded that OPDIVO® is highly active with favorable toxicities in heavily pretreated relapsed or refractory Hodgkin's lymphoma “highlighting the genetically defined sensitivity to PD-1 blockade”. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. Ansell SM, Lesokhin AM, Borrello I, et al. N Engl J Med 2015; 372:311-319
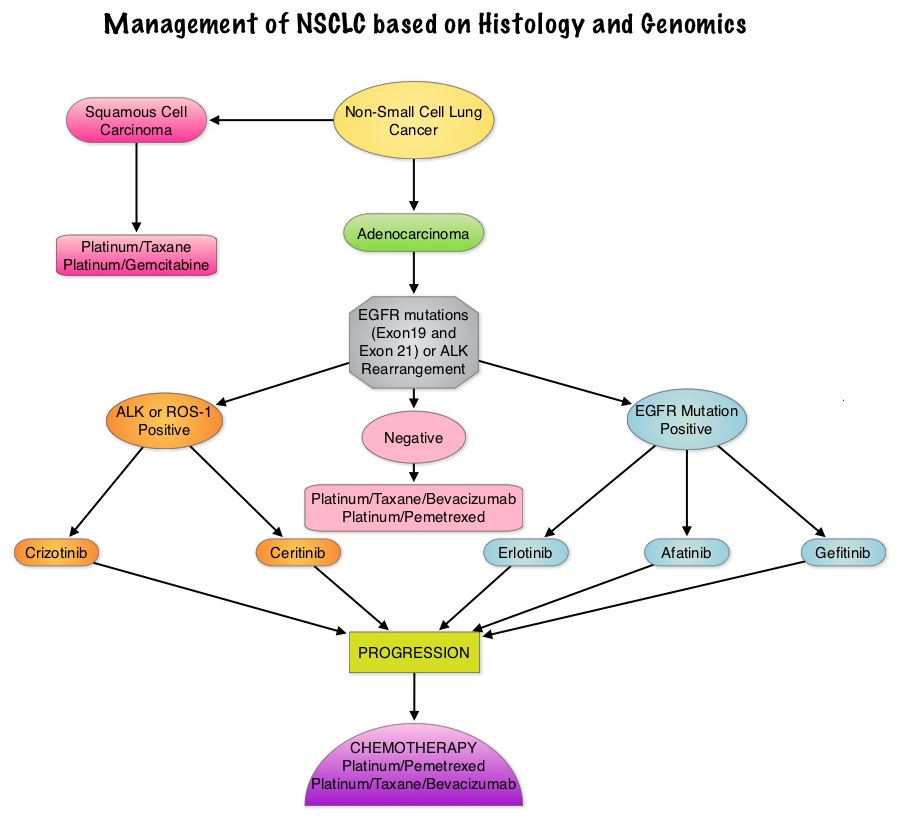 Approximately 1% – 2% of lung adenocarcinomas harbor ROS1 gene rearrangements. ROS1 gene is located on chromosome 6q22 (long arm of chromosome 6) and plays an important role in cell growth and development. ROS1 gene fusion with another gene results in a mutated DNA sequence which then produces an abnormal protein responsible for unregulated cell growth and cancer. ROS1 gene rearrangement has been identified as a driver mutation in Non Small cell Lung Cancer with adenocarcinoma histology. This is more common in nonsmokers or in light smokers (<10 pack years) who are relatively young (average age of 50 years) and thus share similar characteristics with ALK-positive patients. The ROS protein and the ALK protein have similar structure and function and are sensitive to Tyrosine Kinase Inhibitors such as XALKORI® (Crizotinib) and ZYKADIA® (Ceritinib). ROS1 mutations have been also been associated with Cholangiocarcinoma (Bile duct cancer) and Glioblastoma multiforme. ROS1 rearrangements are mutually exclusive with other oncogenic mutations found in NSCLC such as EGFR mutations, KRAS mutations and ALK rearrangement. The presence of a ROS1 rearrangement can be detected by Fluorescence In Situ Hybridization (FISH), ImmunoHistoChemistry (IHC), Reverse Transcriptase– Polymerase Chain Reaction (RT-PCR) and Next Generation-Sequencing. XALKORI® is a small molecule Tyrosine Kinase Inhibitor that targets ALK, MET and ROS1 tyrosine kinases. In a previously published expansion cohort of the phase 1 study by Shaw and colleagues ( NEJM 2014; 371:1963-1971), XALKORI® showed significant activity in patients with in patients with advanced ROS1rearranged NSCLC. The authors in this publication provided additional evidence that ROS1 gene rearrangement is an actionable target in NSCLC, by conducting a retrospective study in centers that tested for ROS1 rearrangement and evaluated the outcomes of ROS1-positive NSCLC patients, who had been treated with XALKORI®. They included 32 patients with NSCLC whose tumors showed ROS1 rearrangement and who had received off-label treatment with XALKORI®. The median age was 50.5 years. They noted an overall response rate of 80% and a disease control rate, 86.7%. The median Progression Free Survival (PFS) was 9.1 months, and the PFS rate at 12 months was 44%. This impressive efficacy data again validates that similar to EGFR mutations and ALK rearrangements, ROS1 gene rearrangements are molecular drivers and patients with NSCLC with adenocarcinoma histology should be tested for ROS1. Crizotinib Therapy for Advanced Lung Adenocarcinoma and a ROS1 Rearrangement: Results From the EUROS1 Cohort. Mazières J, Zalcman G, Crinò L, J Clin Oncol. 2015;33:992-999
Approximately 1% – 2% of lung adenocarcinomas harbor ROS1 gene rearrangements. ROS1 gene is located on chromosome 6q22 (long arm of chromosome 6) and plays an important role in cell growth and development. ROS1 gene fusion with another gene results in a mutated DNA sequence which then produces an abnormal protein responsible for unregulated cell growth and cancer. ROS1 gene rearrangement has been identified as a driver mutation in Non Small cell Lung Cancer with adenocarcinoma histology. This is more common in nonsmokers or in light smokers (<10 pack years) who are relatively young (average age of 50 years) and thus share similar characteristics with ALK-positive patients. The ROS protein and the ALK protein have similar structure and function and are sensitive to Tyrosine Kinase Inhibitors such as XALKORI® (Crizotinib) and ZYKADIA® (Ceritinib). ROS1 mutations have been also been associated with Cholangiocarcinoma (Bile duct cancer) and Glioblastoma multiforme. ROS1 rearrangements are mutually exclusive with other oncogenic mutations found in NSCLC such as EGFR mutations, KRAS mutations and ALK rearrangement. The presence of a ROS1 rearrangement can be detected by Fluorescence In Situ Hybridization (FISH), ImmunoHistoChemistry (IHC), Reverse Transcriptase– Polymerase Chain Reaction (RT-PCR) and Next Generation-Sequencing. XALKORI® is a small molecule Tyrosine Kinase Inhibitor that targets ALK, MET and ROS1 tyrosine kinases. In a previously published expansion cohort of the phase 1 study by Shaw and colleagues ( NEJM 2014; 371:1963-1971), XALKORI® showed significant activity in patients with in patients with advanced ROS1rearranged NSCLC. The authors in this publication provided additional evidence that ROS1 gene rearrangement is an actionable target in NSCLC, by conducting a retrospective study in centers that tested for ROS1 rearrangement and evaluated the outcomes of ROS1-positive NSCLC patients, who had been treated with XALKORI®. They included 32 patients with NSCLC whose tumors showed ROS1 rearrangement and who had received off-label treatment with XALKORI®. The median age was 50.5 years. They noted an overall response rate of 80% and a disease control rate, 86.7%. The median Progression Free Survival (PFS) was 9.1 months, and the PFS rate at 12 months was 44%. This impressive efficacy data again validates that similar to EGFR mutations and ALK rearrangements, ROS1 gene rearrangements are molecular drivers and patients with NSCLC with adenocarcinoma histology should be tested for ROS1. Crizotinib Therapy for Advanced Lung Adenocarcinoma and a ROS1 Rearrangement: Results From the EUROS1 Cohort. Mazières J, Zalcman G, Crinò L, J Clin Oncol. 2015;33:992-999
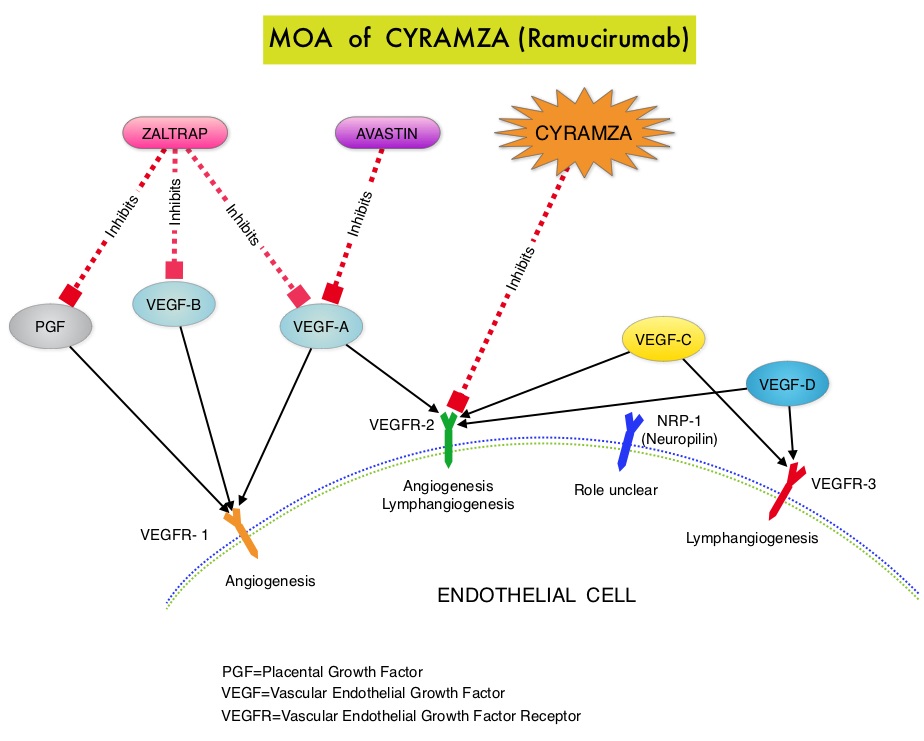 CYRAMZA® (Ramucirumab) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN®, which inhibits VEGF-A. Several studies have demonstrated that continuing VEGF inhibition along with standard second-line chemotherapy beyond disease progression has a significant advantage, in patients with mCRC. Two observational studies (BRiTE and ARIES) as well as an open label phase III study have all shown improvement in median Overall Survival (OS) when AVASTIN® was continued beyond first progression and given along with second line chemotherapy. In the VELOUR trial, FOLFIRI in combination with ZALTRAP® (Aflibercept), a VEGF-A, VEGF-B and Placental Growth Factor inhibitor, when given as second line therapy for those patients with mCRC who had progressed on AVASTIN® plus ELOXATIN® containing regimen, resulted in a significant improvement in the median PFS and OS.
CYRAMZA® (Ramucirumab) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN®, which inhibits VEGF-A. Several studies have demonstrated that continuing VEGF inhibition along with standard second-line chemotherapy beyond disease progression has a significant advantage, in patients with mCRC. Two observational studies (BRiTE and ARIES) as well as an open label phase III study have all shown improvement in median Overall Survival (OS) when AVASTIN® was continued beyond first progression and given along with second line chemotherapy. In the VELOUR trial, FOLFIRI in combination with ZALTRAP® (Aflibercept), a VEGF-A, VEGF-B and Placental Growth Factor inhibitor, when given as second line therapy for those patients with mCRC who had progressed on AVASTIN® plus ELOXATIN® containing regimen, resulted in a significant improvement in the median PFS and OS. When a smoker inhales through the mouth piece of an E-cigarette, the air flow triggers a sensor that switches on a small lithium battery powered heater, which in turn vaporizes liquid nicotine along with PolyEthylene Glycol (PEG) present in a small cartridge. The PEG vapor looks like smoke. The potent liquid form of nicotine extracted from tobacco is tinctured with fragrant flavors such as chocolate, cherry and bubble gum, coloring substances, as well other chemicals and these e-liquids are powerful neurotoxins. With the rapid growth of the E-cigarette industry and the evidence of potential dangers and risk to public health, particularly children, experts from the world's leading lung organizations were compelled to release a position statement on electronic cigarettes, specifically focusing on their potential adverse effects on human health and calling on government organizations to ban or restrict the use of E-cigarettes, until their impact on health is better understood. According to the National Youth Tobacco Survey, the use of e-cigarettes has tripled from 2013 to 2014 among middle school and high school students. Epidemiological data have shown that nicotine use is a gateway to the use of cocaine and marijuana and subsequent lifelong addiction. E-cigarettes and other Electronic Nicotine Delivery Systems (ENDS), unlike combustible cigarettes and many other tobacco products are not currently regulated by the U.S. Food and Drug Administration.
When a smoker inhales through the mouth piece of an E-cigarette, the air flow triggers a sensor that switches on a small lithium battery powered heater, which in turn vaporizes liquid nicotine along with PolyEthylene Glycol (PEG) present in a small cartridge. The PEG vapor looks like smoke. The potent liquid form of nicotine extracted from tobacco is tinctured with fragrant flavors such as chocolate, cherry and bubble gum, coloring substances, as well other chemicals and these e-liquids are powerful neurotoxins. With the rapid growth of the E-cigarette industry and the evidence of potential dangers and risk to public health, particularly children, experts from the world's leading lung organizations were compelled to release a position statement on electronic cigarettes, specifically focusing on their potential adverse effects on human health and calling on government organizations to ban or restrict the use of E-cigarettes, until their impact on health is better understood. According to the National Youth Tobacco Survey, the use of e-cigarettes has tripled from 2013 to 2014 among middle school and high school students. Epidemiological data have shown that nicotine use is a gateway to the use of cocaine and marijuana and subsequent lifelong addiction. E-cigarettes and other Electronic Nicotine Delivery Systems (ENDS), unlike combustible cigarettes and many other tobacco products are not currently regulated by the U.S. Food and Drug Administration.