SUMMARY: The American Cancer Society estimates that in the United States, for the year 2015, approximately 140,000 new cases of ColoRectal Cancer (CRC) will be diagnosed and close to 50,000 patients will die of the disease. The lifetime risk of developing ColoRectal Cancer is about 1 in 20 (5%). Lynch Syndrome (Hereditary NonPolyposis Colorectal Cancer – HNPCC), is an Autosomal Dominant, inherited disorder, associated with an increased risk of colorectal, endometrial, ovary, gastric, small bowel, pancreatic, brain, ureter or renal pelvis cancer. Approximately 3 to 5 percent of all cases of ColoRectal Cancer, are caused by Lynch Syndrome (LS) and 1 in 35 patients with newly diagnosed ColoRectal Cancer is related to Lynch Syndrome. Four MMR genes (MisMatch Repair genes), MLH1, MSH2, MSH6, and PMS2 are involved in the repair of mistakes that occur during DNA replication. When any of these genes are mutated, repair of DNA replication mistakes is prevented resulting in continuous division of abnormal cells and possibly cancer. The EPCAM gene lies next to the MSH2 gene on chromosome 2 and mutations in the EPCAM gene can cause the MSH2 gene to be inactivated, interrupting DNA repair and leading to accumulation of DNA replication errors and possible malignancy. Germline mutations in the MMR genes, is classically seen in Lynch Syndrome and results in microsatellite instability in tumors. Tumors are described as MSI-High when they have changes in 2 or more, of the 5 microsatellite markers. So, high levels of MSI within a tumor are suggestive of defective DNA mismatch repair. MSI-H is a hallmark of Lynch syndrome. However MSI-H is present in approximately 15% of patients with sporadic CRC. This is secondary to epigenetic silencing of MLH1 through promoter hypermethylation, rather than germline mutations in the MMR genes.
A Clinical Diagnosis of Lynch Syndrome can be made based on personal and family history if at least three relatives have a malignancy associated with Lynch Syndrome such as colorectal, endometrial, small bowel, ureter or renal pelvis cancer. In addition the following criteria should be met: • One relative must be a first-degree relative of the other two. • At least two successive generations must be affected. • At least one relative with a Lynch syndrome associated cancer should be diagnosed before 50 years of age. • Familial Adenomatous Polyposis should be excluded. • Tumors should be verified whenever possible. Because family history can sometimes be difficult to obtain or confirm, NCCN in those circumstances has recommended screening all newly diagnosed colorectal cancer patients for Lynch syndrome.
ImmunoHistoChemistry (IHC) staining can be performed on the tumor tissue for protein expression of the four MMR genes. IHC test is described as normal when all 4 mismatch repair proteins are normally expressed suggesting that an underlying mismatch repair gene mutation is unlikely. When IHC test is abnormal, it means that that at least one of the 4 mismatch repair proteins is not expressed and an inherited mutation may be present in the gene related to that protein. This can be further confirmed by mutation analysis of the corresponding gene. However, the lack of expression of MMR proteins by IHC is highly concordant with molecular MSI testing and IHC is therefore more practical and cost-effective. Tumors with loss of MMR protein expression or MSI-H are classified as MMR deficient (dMMR). Patients with sporadic tumors with MSI-H or MMR deficiency (dMMR) generally are older women with stage II disease and present with tumors in the proximal colon and the tumors are poor differentiated with increased number of tumor-infiltrating lymphocytes. These patients in retrospective studies had superior stage-adjusted survival compared to MMR proficient tumors. Further, single agent 5-Fluorouracil when given in an adjuvant setting was not beneficial in this patient group.
It should also be noted that a majority of colon cancer tumors that lack protein expression on IHC staining of MLH1 (often coexisting with loss of PMS2) are often due to an acquired genetic defect. If the IHC indicates absence of MLH1 protein expression, tumor should be tested for BRAF mutation V600E, which can be seen in sporadic colorectal cancers, but rarely found in patients who have Lynch Syndrome. Once a diagnosis of Lynch Syndrome is made, at risk family members should undergo colonoscopic evaluation at 20-25 years of age or 2-5 years prior to the earliest colon cancer, if it is diagnosed before age 25 and is repeated every 1-2 years. Prophylactic hysterectomy and bilateral salpingo-oophorectomy (BSO) should be considered by women who have completed childbearing. NCCN Guidelines Version 1.2014 Lynch Syndrome


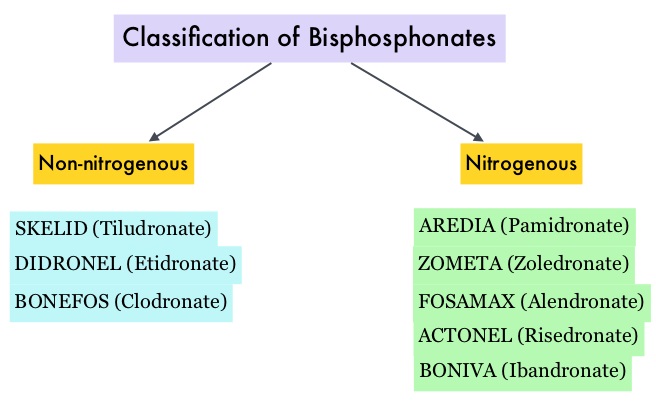 Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.
Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.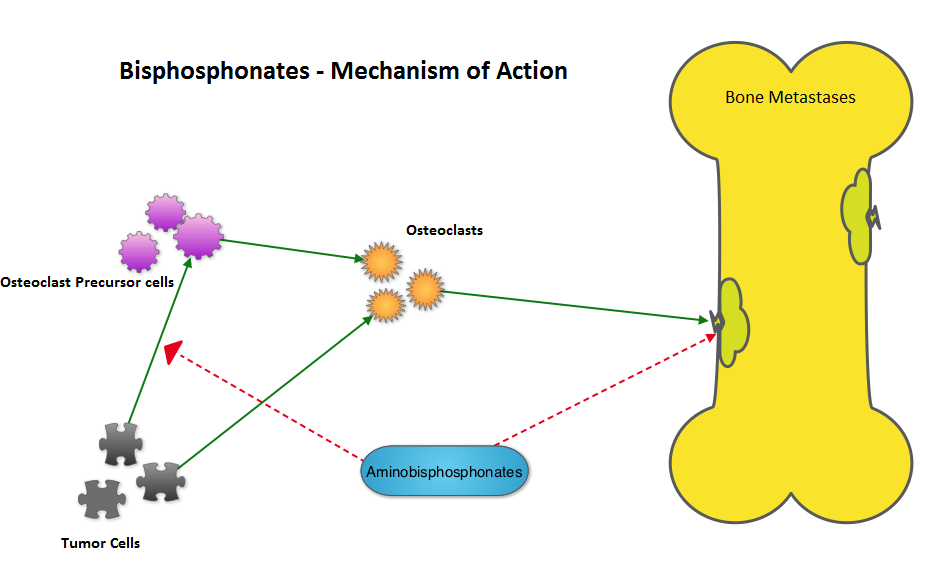 The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.
The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.