SUMMARY: The FDA on September 30, 2015 granted accelerated approval to OPDIVO® (Nivolumab) in combination with YERVOY® (Ipilimumab), for the treatment of patients with BRAF V600 wild-type, unresectable or metastatic melanoma. The American Cancer Society’s estimates that for 2015, approximately 74,000 new melanomas will be diagnosed in the United States and about 10,000 people are expected to die of the disease. The understanding of the Immune checkpoints has lead to the development of novel immunotherapies. Immune checkpoints or gate keepers are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells may be related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), PD-1(Programmed cell Death-1), etc. By doing so, the T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response. YERVOY® is a fully human immunoglobulin G1 monoclonal antibody, that blocks Immune checkpoint protein/receptor CTLA-4 and has been shown to prolong Overall Survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response. OPDIVO® significantly improves Objective Response Rates, in patients with advanced melanoma. Previously published phase I studies demonstrated high Objective Response Rates, including Complete Responses, among patients with advanced melanoma, when treated with a combination of YERVOY® and OPDIVO®.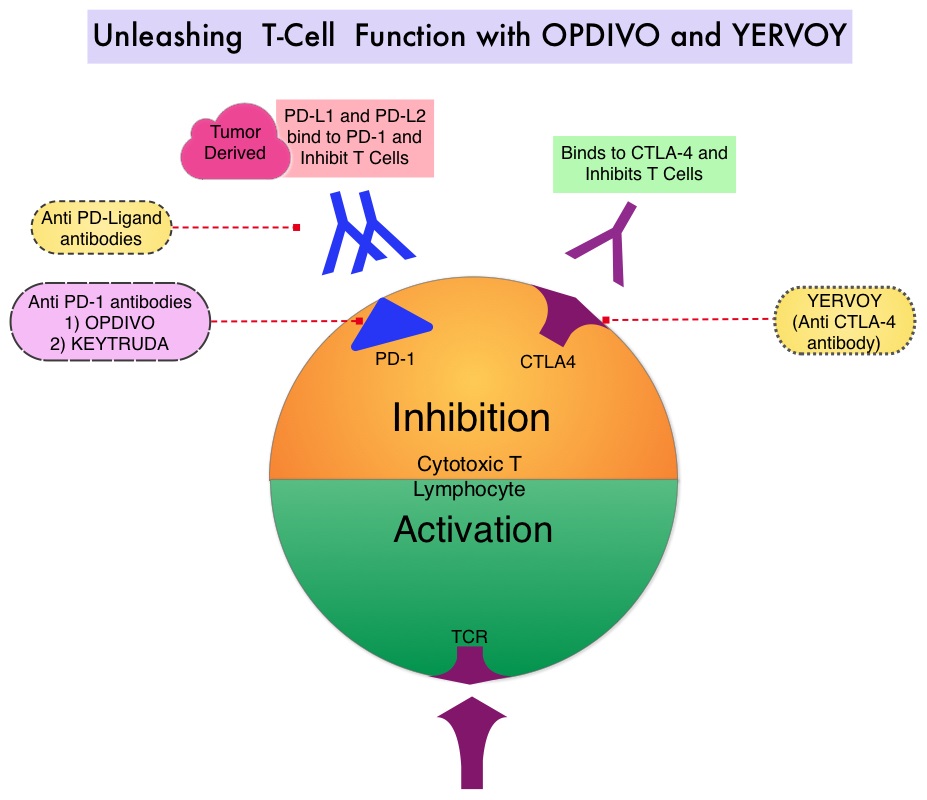
In this double-blind phase II trial, 142 treatment naïve patients with metastatic melanoma were randomly assigned in a 2:1 ratio to receive either OPDIVO® in combination with YERVOY® (N=95) or YERVOY® plus placebo (N=47). Patients in the OPDIVO® plus YERVOY® group received OPDIVO® 1 mg/kg and YERVOY® 3 mg/kg IV every 3 weeks for four doses followed by OPDIVO® 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. Patients in the YERVOY® and placebo group received YERVOY® 3 mg/kg and placebo IV every 3 weeks for four doses followed by placebo. At the time of disease progression, patients in the YERVOY® group were offered OPDIVO® 3 mg/kg every 2 weeks. Patients were stratified according to BRAF mutation status (wild type versus mutation-positive). The median age was 65 years and majority of patients were males. The primary end point was Objective Response Rate among patients with BRAF V600 wild type tumors. The authors noted that in BRAF wild type tumors, the overall Response Rate was 61% with the combination treatment versus 11% for YERVOY® alone (P<0.001). The Complete Response rate was 22% for the combination therapy vs 0% for YERVOY®. The median duration of response was not reached in either treatment groups, with an ongoing response rate of 82% in the combination group and 75% in the YERVOY® plus placebo group. The median Progression Free Survival was not reached with the combination therapy and was 4.4 months in the YERVOY® plus placebo group (HR=0.40; P<0.001). Among patients with BRAF mutation-positive tumors, the overall Response Rate was 52% for the combination therapy group and Complete Response rate was 22%, with similar efficacy compared with the BRAF wild type tumor group. The median Progression Free Survival was 8.5 months for the combination therapy group and 2.7 months for YERVOY® plus placebo group.
Grade 3 or 4 adverse events were reported in 54% of the patients who received the combination therapy as compared with 24% of the patients who received YERVOY® monotherapy and the most common grade 3 or 4 toxicities in the combination therapy group were colitis, diarrhea and elevated transaminases, which resolved with steroids. The authors concluded that a combination of YERVOY® and OPDIVO® is superior to YERVOY® monotherapy, among treatment naïve patients with metastatic melanoma, with significantly greater objective response rate, as well as Progression Free Survival. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. Postow MA, Chesney J, Pavlick AC, et al. N Engl J Med 2015; 372:2006-2017