SUMMARY: The American Cancer Society estimates that in 2016, over 53,000 people will be diagnosed with pancreatic cancer in the United States and close to 42,000 patients will die of the disease. Some important risk factors for Pancreatic cancer include increasing age, obesity, smoking history, genetic predisposition, exposure to certain dyes and chemicals, heavy alcohol use and pancreatitis. The best chance for long term survival is complete surgical resection, although this may not be feasible in a majority of the patients, as they present with advanced disease at the time of diagnosis. Based on the National Cancer Data Base, the 5 year observed survival rate for patients diagnosed with exocrine cancer of the Pancreas is 14% for those with Stage IA disease and 1% for those with Stage IV disease.
ONIVYDE® is a novel nanoliposomal encapsulation of Irinotecan, a topoisomerase 1 inhibitor. It is designed to optimize the delivery of Irinotecan, by extending the duration of circulation of the drug in the body and preferentially activating the drug within the tumor tissues, to achieve higher levels of the active cytotoxic drug metabolite, SN-38. This approach reduces the toxicity of Irinotecan to normal tissues while maintaining or increasing its anti-tumor efficacy.
NAPOLI-1 is an open-label phase III study in which 417 patients with Gemcitabine-refractory metastatic Pancreatic adenocarcinoma were randomly assigned in a 1:1:1 ratio to receive either ONIVYDE® monotherapy, ONIVYDE® plus 5-FluoroUracil (5-FU) and Leucovorin or 5-FU with Leucovorin (control group). Sixty one percent (61%) of patients had cancer in the head of the Pancreas and 68% had liver metastases. Treatment consisted of ONIVYDE® 120 mg/m2 IV over 90 minutes every 3 weeks in Group A, ONIVYDE® 80 mg/m2 IV given over 90 minutes followed by 5-FU 2400 mg/m2 given over 46 hours and racemic Leucovorin 400 mg/m2 IV given over 30 minutes every 2 weeks in Group B and 5-FU 2000 mg/m2 IV given over 24 hours plus racemic Leucovorin 200 mg/m2 IV given over 30 minutes weekly for 4 weeks followed by 2 weeks of rest in Group C (Control group). Each of the two ONIVYDE® containing groups was compared with the 5FU/Leucovorin control group. The primary study endpoint was Overall Survival and secondary endpoints included Progression Free Survival (PFS) and Overall Response Rate (ORR).
The primary survival analysis was previously reported was based on 313 events. The combination of ONIVYDE®, 5-FU and Leucovorin resulted in a median OS of 6.1 months compared with 4.2 months with 5-FU and Leucovorin alone (HR = 0.67; P=0.012). The median PFS was 3.1 months for the ONIVYDE® combination compared with 1.5 months with 5-FU and Leucovorin alone (HR= 0.55; P=0.0001). The ORR was low in both treatment groups (7.7% vs 0.8%), respectively. ONIVYDE® montherapy was not superior, compared with 5-FU and Leucovorin and was associated with more side effects compared to the combination regimen. In an expanded, pre-specified analyses, patients who received at least 80% of the target dose in the first 6 weeks experienced an even greater Overall Survival benefit (43% improvement) with ONIVYDE® combination, compared with 5-FU and Leucovorin alone (8.9 months vs 5.1 months, HR=0.57, P=0.011).
This publication is an updated analysis of OS along with 6 and 12 month survival estimates and safety, based on 378 events, as of 25 May 2015. The authors noted that that the combination of ONIVYDE®, 5-FU and Leucovorin maintained its median OS of 6.2 months compared with 4.2 months with 5-FU and Leucovorin alone (HR = 0.75; P=0.04). Again, there was no OS advantage with ONIVYDE® monotherapy, when compared with 5-FU and Leucovorin. The 6 month survival estimate was 53% with ONIVYDE®, 5-FU and Leucovorin compared with 38% for 5-FU and Leucovorin alone and 12 month survival estimates were 26% for ONIVYDE®, 5-FU and Leucovorin versus 16% for 5-FU and Leucovorin alone. The impact of ONIVYDE® on Overall Survival was more dramatic, with increasing benefit seen with higher levels of CA 19-9. The most common grade 3/4 adverse events with ONIVYDE® plus 5-FU and Leucovorin were neutropenia, fatigue, diarrhea and vomiting.
The authors following this updated analysis concluded that the median Overall Survival benefit for ONIVYDE® plus 5-FU and Leucovorin was maintained, with no new adverse events and this combination may be a new standard of care for patients with metastatic Pancreatic cancer, previously treated with Gemcitabine based therapy. Updated overall survival analysis of NAPOLI-1: Phase III study of nanoliposomal irinotecan (nal-IRI, MM-398), with or without 5-fluorouracil and leucovorin (5-FU/LV), versus 5-FU/LV in metastatic pancreatic cancer (mPAC) previously treated with gemcitabine-based therapy. Wang-Gillam A, Li C, Bodoky G, et al. J Clin Oncol 34, 2016 (suppl 4S; abstr 417)

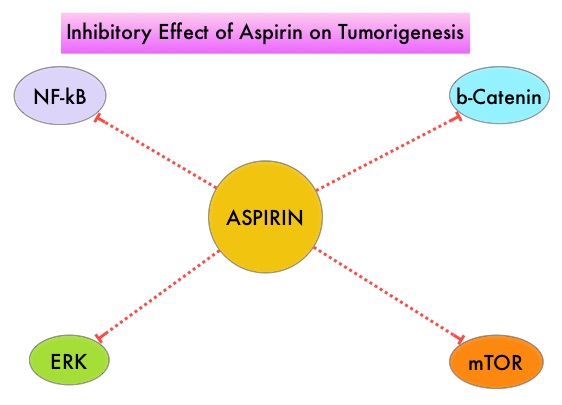
 Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC (after the diagnosis of CRC) is unclear. Platelets have long been implicated in the mechanism of tumor metastases. More recent data suggests that platelets may play a role in tumorigenesis as well, through the release of angiogenic and growth factors due to overexpression of COX-2. Daily low dose Aspirin inhibits COX-1 and COX-2. It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties.
Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC (after the diagnosis of CRC) is unclear. Platelets have long been implicated in the mechanism of tumor metastases. More recent data suggests that platelets may play a role in tumorigenesis as well, through the release of angiogenic and growth factors due to overexpression of COX-2. Daily low dose Aspirin inhibits COX-1 and COX-2. It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties.