SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that 252,710 new cases of invasive breast cancer and 63,410 new cases of non-invasive breast cancer will be diagnosed in women in 2017 and 40,610 women are expected to die from the disease. It is estimated that approximately 140 million women worldwide use hormonal contraception and this number accounts for approximately 13% of women between the ages of 15 and 49 years. The use of hormonal contraception has been on the rise.
Estrogen promotes the development of breast cancer. Previous published studies had shown positive associations between the use of oral contraceptives and breast cancer risk, but the results have been inconsistent from no increase in risk to a 20-30% increase in risk. Further, unlike contemporary hormonal contraception, oral contraceptives in the past contained a higher estrogen dose in the combined estrogen/progestin hormonal contraceptives, and the higher dose of estrogen has been implicated in the development of breast cancer. Contemporary products with new progestins and new routes of delivery (intrauterine system, contraceptive patches, vaginal rings, progestin-only implants, and injections) has raised new concerns, with some studies suggesting that the addition of progestin appears to increase the risk of breast cancer among postmenopausal women who receive hormone therapy. There is evidence to suggest that use of hormonal contraception at a young age may confer a higher risk of breast cancer than initiation of use later in life.
This Danish study, using their national registry, reported the risk of invasive breast cancer risk among women of reproductive age, who were using currently available hormonal contraception. This prospective cohort study conducted in Denmark included 1.8 million women between 15-49 years of age, who did not have a diagnosis of cancer or venous thromboembolism, and who had not received treatment for infertility. Individually updated information about the use of hormonal contraception, breast cancer diagnoses, and potential contributing factors, was obtained from nationwide registries.
It was noted that when compared with women who had never used hormonal contraception, the relative risk of breast cancer among all current and recent users of hormonal contraception was 1.20 (20% higher than average). This risk increased from 1.09 (9% higher than average) with less than 1 year of use to 1.38 (38% higher than average) with more than 10 years of hormonal contraception use (P=0.002). After discontinuation of hormonal contraception, the risk of breast cancer continued to be higher among the women who had used hormonal contraceptives for 5 years or more than among women who had not used hormonal contraceptives. Women who currently or recently used the progestin-only intrauterine system also had a higher risk of breast cancer than women who had never used hormonal contraceptives, with a relative risk of 1.21 (21% higher than average). Outcomes in this study however, could not be adjusted for age at menarche, breast feeding, alcohol consumption, physical activity or Body Mass Index.
It was concluded from this large study population that, the risk of breast cancer was higher among women who currently or recently used contemporary hormonal contraceptives than among women who had never used hormonal contraceptives, and this risk increased with longer durations of use. Additionally, these results unequivocally suggest that no hormonal contraceptives are free of risk. Contemporary Hormonal Contraception and the Risk of Breast Cancer. Mørch LS, Skovlund CW, Hannaford PC, et al. N Engl J Med 2017; 377:2228-2239

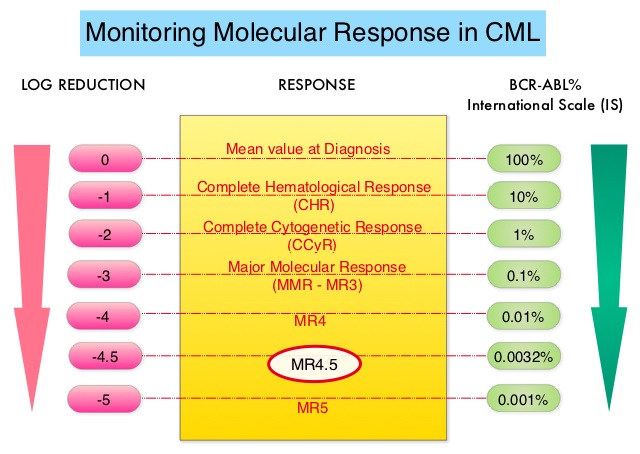 This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241).
This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241).