SUMMARY: The American Cancer Society estimates that in 2018, about 55,440 people will be diagnosed with pancreatic cancer and about 44,330 people will die of the disease. Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States and Western Europe. Unfortunately, unlike other malignancies, very little progress has been made and outcomes for patients with advanced pancreatic cancer, has been dismal. Diagnosis is often made late in the course of the disease, as patients are often asymptomatic and early tumors cannot be detected during routine physical examination. Research has been underway evaluating the role of modifiable risk factors, early screening biomarkers, and tumor microenvironment and their influence on outcomes.
Recent published studies have shown the influence of gut microbiome alterations, in the carcinogenesis of pancreatic cancer. A commensal microbiome in a healthy individual maintains a symbiotic relationship conferring protection by its inflammatory-modulating activity, detoxification, hormonal homeostatic and metabolic effects of bacterial metabolites. An imbalanced microbiome can result in dysbiosis, and microbiome alteration has been reported to contribute to carcinogenesis of multiple malignancies. One classic example of influence of microbiome alteration contributing to carcinogenesis is Helicobacter pylori (H. pylori). Eradication of H. pylori causes regression of MALT lymphoma and decreases risk of metachronous gastric carcinoma after endoscopic resection for early stage gastric cancer.
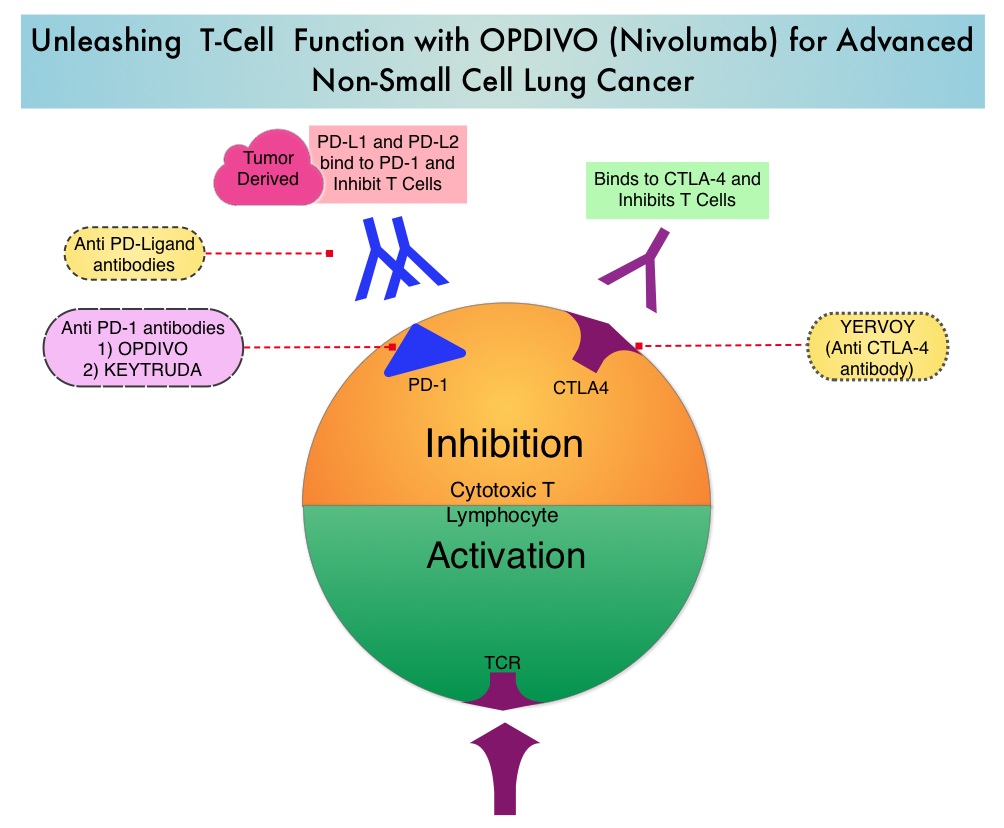
An abundance and alteration of certain microbiomes has been shown to suppress monocytic cellular differentiation in pancreatic cancer leading to T-cell anergy. Targeting the microbiome and bacterial ablation has been shown to be associated with immunogenic reprogramming of the pancreatic tumor microenvironment, by reducing the myeloid-derived suppressor cells and increasing macrophage differentiation. Additionally, bacterial ablation also upregulates PD-1 expression, and enables the efficacy for checkpoint-based immunotherapy and reverses intratumoral immune tolerance. These findings have led to the conclusion that, endogenous microbiota promote immune suppression, characteristic of pancreatic ductal adenocarcinoma, and targeting microbiome potentially can modulate disease progression.
The present study was based on preclinical findings that cancerous pancreas harbors a population of bacteria that is a 1000 fold more, compared with normal pancreas in both mice and humans, and these select bacteria are differentially increased in the tumorous pancreas compared with the gut. The authors in this study noted that bacteria that are more abundant in pancreatic cancers include proteobacteria, actinobacteria, and fusobacteria species. These bacteria release cell membrane components such as lipopolysaccharides and proteins such as flagellins that shift macrophages into immune suppression and prevent the immune system from attacking tumor cells. Their study showed that eliminating these bacteria using antibiotics restored the ability of immune cells to recognize cancer cells, slowed pancreatic tumor growth, and reduced the cancer cell tumor burden by 50% in study animals. Specifically, it was noted that eradicating these bacteria from the gut and pancreas, by treating mice with antibiotics, slowed cancer growth and allowed the recognition of tumor cells by the immune system. Oral antibiotics also increased the efficacy of checkpoint inhibitors roughly 3 fold, thereby strongly improving antitumor immunity.
The researchers further pointed out that even though alterations in genes such as KRAS can result in abnormal cell growth and development of pancreatic cancer, the present study showed that bacteria can change the immune environment around cancer cells and facilitate rapid tumor growth in some patients more so than others, despite their similar genetic alterations. The authors hypothesized that changes in the genes that cause abnormal cell growth in the pancreas might also change the immune response, favoring the growth of different bacterial species, other than those found in healthy individuals. Environmental factors like diet, other medical conditions, or common medications, might also cause bacterial changes in the gut, that influence the pancreatic microbiome.
It was concluded that in pancreatic cancer, a distinct and abundant group of bacteria provide an immune suppressed environment, and addition of antibiotics improved the efficacy of a checkpoint inhibitor, in a mouse model of pancreatic ductal adenocarcinoma, as demonstrated by an increase in T cells that could attack the tumor. Studies are planned to evaluate the role of antibiotic combinations such as Ciprofloxacin and Metronidazole and their benefit in improving the efficacy of PD-1 inhibitors, in patients with pancreatic ductal adenocarcinoma. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Pushalkar S, Hundeyin M, Daley D, et al. DOI: 10.1158/2159-8290.CD-17-1134