SUMMARY: Chronic Myeloid Leukemia (CML) constitutes about 15% of all new cases of leukemia. The American Cancer Society estimates that about 8,990 new CML cases will be diagnosed in the United States in 2019 and about 1,140 patients will die of the disease. The hallmark of CML, the Philadelphia Chromosome (Chromosome 22), is a result of a reciprocal translocation between chromosomes 9 and 22, wherein the ABL gene from chromosome 9 fuses with the BCR gene on chromosome 22. As a result, the auto inhibitory function of the ABL gene is lost and the BCR-ABL fusion gene is activated resulting in cell proliferation and leukemic transformation of hematopoietic stem cells.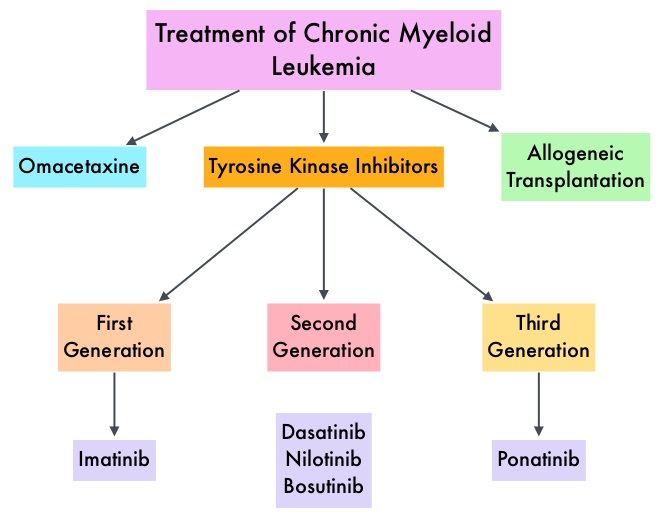
The presently available Tyrosine Kinase Inhibitors (TKI’s) approved in the United States including GLEEVEC® (Imatinib), share the same therapeutic target, which is BCR-ABL kinase. Resistance to TKI’s can occur as a result of mutations in the BCR-ABL kinase domain or amplification of the BCR-ABL gene. With the availability of newer therapies for CML, monitoring response to treatment is important. This is best accomplished by measuring the amount of residual disease using Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Molecular response in CML is expressed using the International Scale (IS) as BCR-ABL%, which is the ratio between BCR-ABL and a control gene. BCR-ABL kinase domain point mutations are detected using the mutational analysis by Sanger sequencing. Majority of the patients receiving a TKI following diagnosis of CML achieve a Complete Cytogenetic Response (CCyR) within 12 months following commencement of therapy and these patients have a life expectancy similar to that of their healthy counterparts. Previously published studies have shown that Deep Molecular Response (BCR-ABL <0.01% on the International Scale – MR4) is a new molecular predictor of long term survival in CML patients, and this was achieved in a majority of patients treated with optimized dose of GLEEVEC®. Further, it has been shown on previous observations, that a subgroup of CML patients experiencing deeper responses (MR3, MR4, and MR4.5), may stay in unmaintained remission even after treatment discontinuation. Despite this observation, precise criteria for stopping CML therapy have not been clearly defined.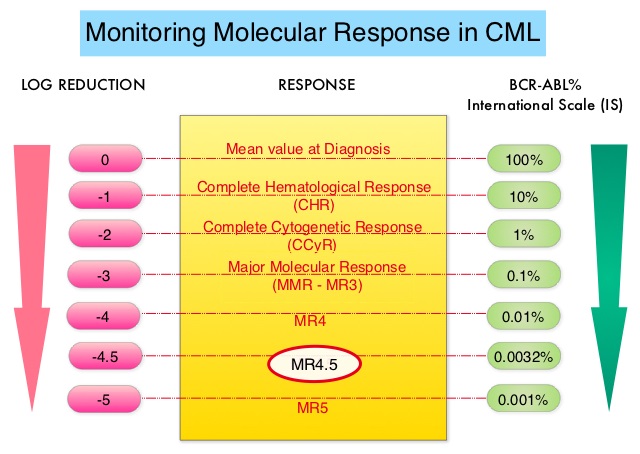
Discontinuing TKI therapy after a Deep Molecular Response among patients with CML can potentially improve quality of life, minimize long term toxicities as well as drug-drug interactions, and reduce financial burden. Two important studies, STIM (Stop Imatinib) and EURO-SKI have set the stage for TKI discontinuation of TKI therapy in CML patients, who are in deep molecular remission, taking into consideration Sokal score at diagnosis, duration on TKI therapy and molecular response based on BCR-ABL transcripts log reduction. Sokal score is calculated using a formula that includes Age, Spleen size, Platelet count and percentage of Myeloblasts and has three risk groups: Low-risk (Sokal score<0.8), Intermediate-risk (Sokal score 0.8-1.2) and High-risk (Sokal score >1.2).
Stopping TKI therapy among CML patients appears to be safe and feasible in over 50% of the patients, although about 20% of these patients experience TKI withdrawal syndrome manifesting as musculoskeletal symptoms. Discontinuation of TKI therapy should only be considered in consenting patients after a thorough discussion of the potential risks and benefits.
Criteria for TKI Discontinuation: Outside of a clinical trial, TKI discontinuation should be considered only if a patient meets ALL the criteria listed below-
1) Age 18 years or older.
2) Chronic phase CML with no prior history of Accelerated or Blast phase.
3) On approved TKI therapy for at least 3 years.
4) Prior evidence of quantifiable BCR-ABL1 transcript.
5) Stable molecular response defined as MR4, (BCR-ABL equal to 0.01% or less IS), for 2 or more years as documented on at least 4 tests, performed at least 3 months apart.
6) Access to qPCR test that can reliably detect at least MR4.5 (BCR-ABL equal to 0.0032% or less IS), with results available within 2 weeks.
7) For patients who remain in Major Molecular Remission or MMR (MR3, BCR-ABL equal to 0.1% or less IS) after discontinuation of TKI therapy, the recommendations are monthly molecular monitoring the first year, every 6 weeks the second year and every 12 weeks thereafter, indefinitely.
8) TKI therapy should be promptly resumed within 4 weeks of a loss of MMR, with molecular monitoring every 4 weeks until MMR is re-established and then every 12 weeks thereafter, indefinitely. If a patient fails to achieve MMR after 3 months of TKI resumption, BCR-ABL kinase domain mutation testing should be performed, and monthly molecular monitoring should be continued for an additional 6 months.
9) Consultation with a CML Specialty Center is recommended regarding the appropriateness for TKI discontinuation, and potential risks and benefits of discontinuing therapy, including TKI withdrawal syndrome.
10) It is strongly encouraged to report the following to an NCCN CML Panel Member-
a) Any significant adverse event thought to be related to therapy discontinuation.
b) Progression to Accelerated or Blast phase at any time.
c) Failure to regain MMR after 3 months following treatment reinitiation.
NCCN guidelines updates: discontinuing TKI therapy in the treatment of chronic myeloid leukemia. Shah NP. Presented at 2019 NCCN Annual Conference; March 21-23, 2019; Orlando, FL.