There is an approximately 30% increased risk of breast cancer recurrence or death in those who are obese, compared to those with ideal body weight. Obesity is associated with alterations in Insulin/glucose homeostasis, adipokines, and sex hormones, which may all play a role in breast cancer outcomes.
BMI (Body Mass Index) does not discriminate between adiposity and muscle, and individuals deemed healthy based on a normal BMI may still be prone to cardiometabolic disorders due to high levels of visceral fat. It has been reported that approximately 18% of women with normal BMI had excess fat, detected on DEXA scan.
In a recently published article in JAMA Oncology involving 3460 postmenopausal women with normal BMI, there was a 56% increase in the risk of developing ER-positive breast cancer per 5-kg increase in trunk fat, despite a normal BMI. This study concluded that a normal BMI may not be an adequate proxy for the risk of breast cancer in postmenopausal women but high body fat levels and altered levels of circulating metabolic and inflammatory factors may be associated with a higher risk of invasive breast cancer.
Month: April 2019
High Body Fat Level Increases Breast Cancer Risk in Postmenopausal Women with Normal BMI
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 268,600 new cases of female breast cancer will be diagnosed in 2019 and about 41,760 women will die of the disease. Obesity is an important contributing factor to postmenopausal breast cancer incidence and mortality. Based on recently published meta-analysis, in women diagnosed with breast cancer, there is an approximately 30% increased risk of disease recurrence or death in those who are obese, compared to those with ideal body weight. Increasing physical activity may lower the risk of breast cancer recurrence. According to the consensus from the St Gallen Consensus Conference in 2015, obesity has been associated with poor breast cancer outcomes. Obesity is associated with alterations in Insulin/glucose homeostasis, adipokines, and sex hormones, which may all play a role in breast cancer outcomes. Weight loss can lead to reductions in C-reactive protein, Insulin, glucose, and Leptin. These mediators have all been implicated to have prognostic significance in breast cancer.
Body Mass Index (BMI) measures body size and is calculated based on height and weight. It is used as a measure of obesity. BMI however does not discriminate between adiposity and muscle and individuals deemed healthy based on a normal BMI may still be prone to cardiometabolic disorders due to high levels of visceral fat. Dual-energy X-ray absorptiometry (DXA or DEXA) is often utilized to measure bone mineral density and is the most accurate method presently available for the diagnosis of osteoporosis and to estimate fracture risk. DEXA scan can also be used to measure total body composition and fat content including the amount of visceral fat, with a high degree of accuracy by its ability to break down fat, bone and muscle tissue. It has been reported that approximately 18% of women with normal BMI had excess fat, detected on DEXA scan.
There are two types of adipose tissue in the human body, White Adipose Tissue (WAT) and Brown Adipose Tissue (BAT) which have antagonistic functions. White Adipose Tissue or white fat cells represent the body’s main type of fat tissue and each fat cell has a single lipid droplet. They are distributed in the subcutaneous tissue, around a person's waist and thighs and around internal organs (visceral fat). WAT stores excess energy as triglycerides and serves as an energy reservoir. Brown Adipose tissue (BAT) which is abundant in small mammals and in newborns generates heat by burning calories and helps them to survive cold temperatures. Brown adipocytes contain several small lipid droplets, and a high number of iron-containing mitochondria which gives brown fat its dark tan color. Most BAT is distributed in the lower neck and interscapular area of an adult, and above the collarbone. Higher quantities of BAT are associated with lower body weight and BAT decreases and body weight increases with increasing age.
Leptin is a hormone produced primarily by adipose tissue and circulating Leptin levels correlate with the body fat stores, with increased circulating Leptin levels noted in individuals with excess adiposity. Leptin can induce Aromatase which synthesizes estrogen, can directly stimulate cancer cell proliferation and survival, and activate Estrogen Receptor α via ligand-independent mechanism.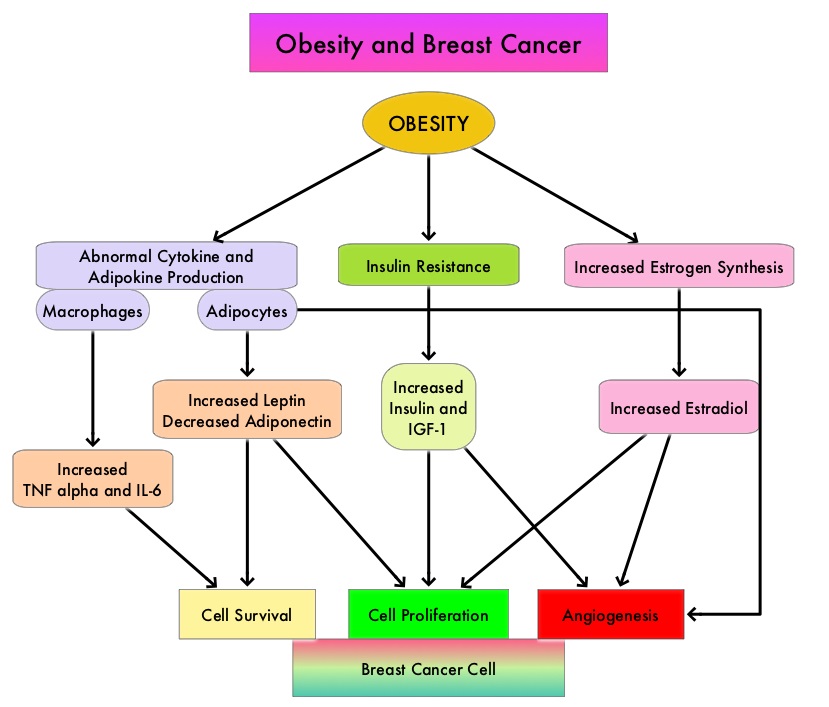
The increased risk of postmenopausal breast cancer in women with normal BMI is poorly understood. Recent studies have shown that in these women with normal BMI, excess body fat is associated with adipocyte hypertrophy which correlates with WAT inflammation, increased circulating Leptin levels, elevated levels of Aromatase and elevated Insulin levels. Dysregulation of Insulin signaling can activate the PI3K/Akt/mTOR and Ras/Raf/MAPK pathways which in turn can enhance cell proliferation and increase the risk of breast cancer. Further, Insulin also induces insulin like growth factor-1 (IGF-1), which can activate ERα. Insulin resistance leads to reduced levels of sex hormone–binding globulin, resulting in elevated levels of free estradiol. It has been suggested that all of these changes collectively may play a role in the pathogenesis of obesity-related breast cancer. The present study was conducted to investigate the association between body fat and breast cancer risk in women with normal BMI.
The authors in this long-term prospective study examined the association between body fat mass, measured by DEXA scan, and the risk of breast cancer, in a secondary analysis of 3460 postmenopausal women with normal BMI (18.5-24.9), enrolled in the Women’s Health Initiative (WHI) clinical trials or observational study. The goal of this study was to understand whether excess adipose tissue is associated with an increased breast cancer risk in women with normal BMI. Participants 50-79 years old with a mean age of 64 years underwent body fat measurement (the percentage of whole-body fat, trunk fat, and fat mass in both legs) with DEXA scan at 3 US designated centers at the time of study entry into the WHI clinical trials, and years 1, 3, 6, and 9. Levels of Insulin, glucose, C-reactive protein, interleukin-6, triglycerides, HDL cholesterol, estradiol, sex hormone-binding globulin, adiponectin, and Leptin were measured in 3-13% of participants using baseline fasting blood specimens.
At a median follow up of 16 years, 182 incident breast cancers were confirmed, and 146 (80%) were ER positive. It was noted that among postmenopausal women with normal BMI, relatively high body fat levels were associated with an elevated risk of invasive breast cancer. The authors specifically, found a 56% increase in the risk of developing ER-positive breast cancer per 5-kg increase in trunk fat, despite a normal BMI. Elevated trunk fat levels were also associated with metabolic dysregulation and inflammation characterized by increased circulating levels of Insulin, Leptin, C-reactive protein, Interleukin 6 and triglycerides, whereas levels of HDL cholesterol and sex hormone–binding globulin were lower.
It was concluded from this large prospective study that normal BMI may not be an adequate proxy for the risk of breast cancer in postmenopausal women. High body fat levels and altered levels of circulating metabolic and inflammatory factors may be associated with a higher risk of invasive breast cancer. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women with Normal Body Mass Index. A Secondary Analysis of a Randomized Clinical Trial and Observational Study. Iyengar NM, Arthur R, Manson JE, et al. JAMA Oncol. 2019;5:155-163
Androgen Suppression for 18 Months plus Radiotherapy is an Effective Treatment Option for Locally Advanced Intermediate and High Risk Prostate Cancer
SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer, and 1 in 9 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 174,650 new cases of Prostate cancer will be diagnosed in 2019 and 31,620 men will die of the disease. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention.
The intergroup trial developed by the NCIC Clinical Trials Group, in collaboration with the Medical Research Council and the National Cancer Institute US Cancer Therapy Evaluation Program, concluded that the addition of radiotherapy to Androgen Suppression significantly prolongs Overall and Disease Specific Survival, in patients with locally advanced prostate cancer. The optimal duration of Androgen Suppression along with radiotherapy, in the curative management of locally advanced prostate cancer however remains unclear. Neoadjuvant Androgen Suppression schedules have ranged in duration from 3-8 months and for those patients with high risk disease, post radiotherapy adjuvant androgen suppression therapy schedules have ranged from 6-36 months. The study published by Bolla, et al. (NEJM 2009;360:2516-2527) concluded that a combination of radiotherapy along with 6 months of Androgen Suppression provided inferior survival compared with radiotherapy plus 3 years of Androgen Suppression, in patients with locally advanced prostate cancer. This long duration of Androgen Suppression however can be associated with significant adverse events. The PCS IV trial (Nabid, et al. European Urology 2018;74: 432-441) compared 36 months of Androgen Suppression and radiotherapy with 18 months of Androgen Suppression and radiation therapy and concluded that there was no difference in survival between the two treatment groups, with the 18-month group experiencing a better quality of life. Zoledronic acid is effective in preventing Androgen Suppression-induced bone loss, but its role in preventing castration-sensitive bone metastases in locally advanced prostate cancer has been unclear. Randomized Androgen Deprivation And Radiotherapy trial (RADAR) was conducted to determine whether an intermediate duration of Androgen Suppression would be superior to short-term Androgen Suppression, without compromising quality of life. This study was also designed to evaluate whether bisphosphonate therapy would help reduce some of the adverse effects associated with Androgen Suppression and prevent bone disease progression. RADAR trial is randomized, Phase III, 2 × 2 factorial study, which was designed to assess whether the addition of 12 months of adjuvant Androgen Suppression, 18 months of zoledronic acid, or both, can improve outcomes in men with locally advanced prostate cancer, who receive 6 months of Androgen Suppression and radiotherapy to the prostate gland.
This trial enrolled 1071 men 18 years or older with locally advanced prostate cancer, defined as either T2b-4, N0 M0 tumors or T2a, N0 M0 tumors with a Gleason score was 7 or more, and baseline PSA of 10 μg/L or more. Patients were randomly assigned in a 1:1:1;1 ratio to four treatment groups, but all patients following randomization received 6 months of neoadjuvant Androgen Suppression with Leuprolide 22.5 mg IM every 3 months, and radiotherapy to the prostate and seminal vesicles five months after randomization. The four treatment groups were 1) STAS or Short-Term Androgen Suppression which was the control group (N=268), in which patients received 6 months of neoadjuvant Androgen Suppression with Leuprolide 22.5 mg IM every 3 months, and radiotherapy to the prostate and seminal vesicles 2) ITAS or Intermediate-Term Androgen Suppression (N=268), in which STAS was followed by an additional 12 months of adjuvant Androgen Suppression (total of 18 months) with Leuprolide 22.5 mg IM every 3 months 3) STAS plus 18 months of Zoledronic acid 4 mg IV every 3 months, starting at randomization (N=268) 4) ITAS plus Zoledronic acid (N=267). The Primary endpoint was prostate cancer-specific mortality. Secondary endpoints included PSA progression, local progression and distant progression. The median follow up was 10.4 years. Because no interactions were observed between Androgen Suppression and Zoledronic acid at this 10 year follow up, treatment groups were collapsed and 6 months of Androgen Suppression (STAS) plus radiotherapy was compared with 18 months of Androgen suppression (ITAS) plus radiotherapy and groups receiving or not receiving treatment with Zoledronic acid, were compared.
It was noted that the use of additional 12 months of adjuvant Androgen Suppression (total of 18 months) resulted in a significant improvement in prostate cancer specific mortality compared to 6 months of Androgen Suppression and radiotherapy (9.7% versus 13.3% respectively), representing an absolute difference of 3.7% (sub-HR=0.70, adjusted P=0.035). The addition of Zoledronic acid did not have an impact on prostate cancer-specific mortality.
The authors concluded that 18 months of Androgen Suppression plus radiotherapy is a more effective treatment option for locally advanced intermediate and high risk prostate cancer patients, than 6 months of Androgen Suppression plus radiotherapy. It was also concluded that the addition of Zoledronic acid to this treatment regimen does not improve outcomes. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Denham JW, Joseph D, Lamb DS, et al. Lancet Oncol 2019;20:267-281
KEYTRUDA® and INLYTA®
The FDA on April 19, 2019, approved KEYTRUDA® (Pembrolizumab) plus INLYTA® (Axitinib) for the first-line treatment of patients with advanced Renal Cell Carcinoma (RCC). KEYTRUDA® is a product of Merck & Co. Inc. and INLYTA® is a product of Pfizer Inc.
FDA Lowers PD-L1 Expression Threshold for KEYTRUDA® and Expands Indication for Frontline Treatment of NSCLC
SUMMARY: The FDA on April 11, 2019, approved KEYTRUDA® (Pembrolizumab) for the first-line treatment of patients with Stage III Non-Small Cell Lung Cancer (NSCLC) who are not candidates for surgical resection or definitive chemoradiation, as well as those with metastatic NSCLC. Patients’ tumors must have no EGFR or ALK genomic aberrations and express PD-L1 (Tumor Proportion Score-TPS of 1% or more), as determined by an FDA-approved test. Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2019 about 228,150 new cases of lung cancer will be diagnosed and 142,670 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response and unleashes the tumor-specific effector T cells. High level of Programmed Death-Ligand 1 (PD-L1) expression is defined as membranous PD-L1 expression on at least 50% of the tumor cells, regardless of the staining intensity. It is estimated that based on observations from previous studies, approximately 25% of the patients with advanced NSCLC have a high level of PD-L1 expression, and high level of PD-L1 expression has been associated with significantly increased response rates to KEYTRUDA®. The FDA approved KEYTRUDA® for the first-line treatment of advanced NSCLC with high PD-L1 expression (Tumor Proportion Score of 50% or more), based on KEYNOTE-024 trial, as well as in combination with Pemetrexed and Carboplatin, as first-line treatment of patients with metastatic non-squamous NSCLC, based on KEYNOTE-021 study. It is also indicated for previously treated advanced NSCLC with a much lower level of PD-L1 expression such as PD-L1 Tumor Proportion Score of 1% or higher, based on KEYNOTE-010 trial.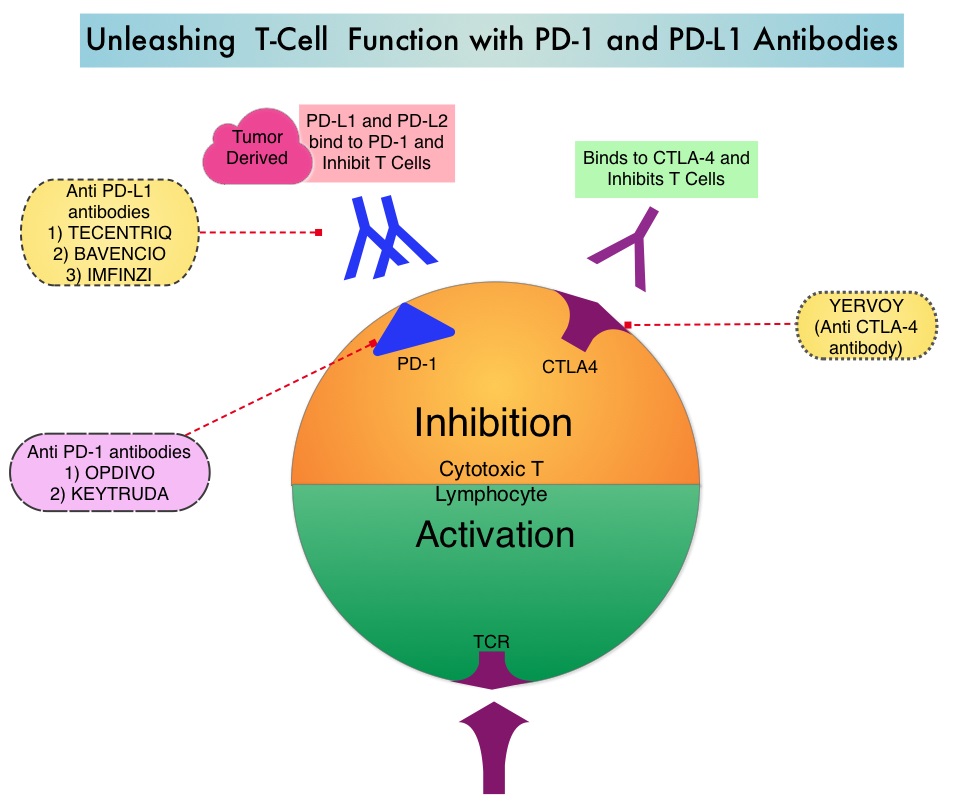
KEYNOTE-042 is a large, international, multicenter, randomized phase III trial in which 1274 patients with untreated locally advanced or metastatic NSCLC were randomly assigned to KEYTRUDA® or chemotherapy with Paclitaxel plus Carboplatin or Pemetrexed plus Carboplatin. In this study, both squamous and non-squamous cancers with PD-L1 Tumor Proportion Score (TPS) of 1% or more were included, but tumors with sensitizing Epidermal Growth Factor Receptor (EGFR) or Anaplastic Lymphoma Kinase (ALK) mutations cancers with genetic changes, that could be treated with targeted therapies such as EGFR and ALK inhibitors, were excluded. Eligible patients were randomly assigned in a 1:1 to receive either KEYTRUDA® 200 mg IV every 3 weeks for up to 35 cycles or investigator’s choice of up to 6 cycles of chemotherapy with Paclitaxel plus Carboplatin or Pemetrexed plus Carboplatin, with optional Pemetrexed maintenance for non-squamous NSCLC. Patients were divided into 3 treatment groups based on their PD-L1 Tumor Proportion Score (TPS): TPS 50% or more (N=599), TPS 20% or more (N=818), and TPS 1% or more (N=637). Each PD-L1 expression group had equal numbers of patients receiving KEYTRUDA® and chemotherapy. The Primary end points were Overall Survival (OS) in patients with TPS 50% or more, 20% or more, and 1% or more.
At a median follow up of 12.8 months, 13.7% of patients were still receiving KEYTRUDA® compared with 4.9% on Pemetrexed maintenance therapy. It was noted that KEYTRUDA® was significantly superior to chemotherapy in all PD-L1 expression subsets. In patients with a PD-L1 TPS 50% or more, the median OS with KEYTRUDA® was 20 months versus 12.2 months for chemotherapy (HR=0.69, P=0.0003), for patients with PD-L1 TPS 20% or more, the median OS was 17.7 months versus 13 months respectively (HR=0.77, P=0.002), and for those with PD-L1 TPS 1% or more, the median OS was 16.7 months versus 12.1 months respectively (HR=0.81, P = 0.0018). The Response Rates (RR) were also higher among patients who received KEYTRUDA®, with RR of 39.5% for KEYTRUDA® versus 32% for chemotherapy in patients with a TPS 50% or more, 33.4% and 28.9% respectively in patients with TPS 20% or more and 27.3% and 26.5%, respectively, among patients with TPS of 1% or more. The duration of response was also superior with KEYTRUDA® in all three PD-L1 subgroups compared to chemotherapy (20.2 months versus 8-11 months). Patients receiving KEYTRUDA® experienced fewer severe Adverse Events, compared with chemotherapy (17.8% versus 41%).
The authors concluded that this is the largest clinical trial of KEYTRUDA® as a stand-alone therapy, and is the first study with a Primary end point of OS to demonstrate superiority of KEYTRUDA® over platinum-based chemotherapy, in patients with previously untreated locally advanced/metastatic NSCLC, without sensitizing EGFR or ALK alterations and a PD-L1 TPS of 1% or more. These data confirmed the benefit of KEYTRUDA® monotherapy as a standard first-line treatment, for PD-L1-expressing locally advanced Stage III as well as metastatic NSCLC. KEYTRUDA® monotherapy is now a new treatment option for more patients with NSCLC, including those for whom combination therapy may not be appropriate. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Mok TS, Wu Y-L, Kudaba I, et al. The Lancet. Published: April 04, 2019. DOI: https://doi.org/10.1016/S0140-6736(18)32409-7
FDA Approves BALVERSA® for Metastatic Urothelial Carcinoma
SUMMARY: The FDA on April 12, 2019 granted accelerated approval to BALVERSA® (Erdafitinib) for patients with locally advanced or metastatic Urothelial Carcinoma, with susceptible FGFR3 or FGFR2 genetic alterations,that has progressed during or following platinum-containing chemotherapy, including within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy. Patients should be selected for therapy based on an FDA-approved companion diagnostic for BALVERSA®. The FDA also simultaneously approved the THERASCREEN® FGFR RGQ RT-PCR Kit, developed by QIAGEN, for use as a companion diagnostic for this therapeutic indication.
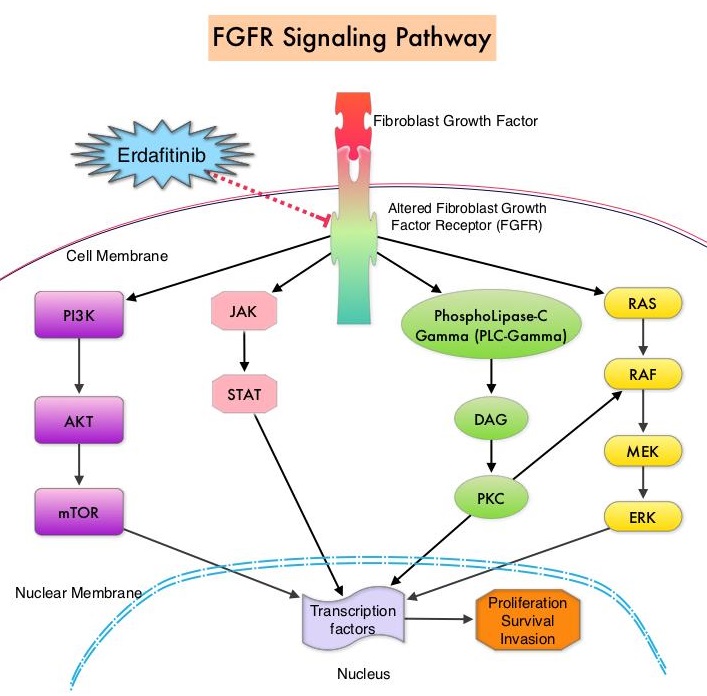 FGFRs are a family of Receptor Tyrosine Kinases, which may be upregulated in a variety of malignancies. The Fibroblast Growth Factor/Fibroblast Growth Factor Receptor (FGF/FGFR) signaling pathway regulates embryogenesis, adult tissue homeostasis, angiogenesis and wound repair, and is also pivotal in cell functions, including proliferation, differentiation, apoptosis and migration. Deregulated FGF/FGFR activations have been associated with developmental disorders and cancer progression. Following binding with a ligand, FGFRs activate downstream signaling pathways such as the Mitogen Activated Protein Kinase (MAPK), Signal Transducer and Activator of Transcription (STAT), the PhosphoInositide-3-Kinase (PI3K)/Akt pathways, and PLC-DAG-PKC pathway. FGFR isoforms have been shown to result in oncogenic FGFR signaling, which in turn promotes tumorigenesis. FGFR3 mutations have been described in approximately 75% of low-grade papillary bladder cancers, and FGFR3 overexpression has been noted in 42% of muscle-invasive bladder cancers. FGFR1 amplification has also been found in 3% of urinary bladder cancers. Patients with FGFR alterations have poor outcomes when treated with available therapies and these alterations occur in 20% of patients with metastatic Urothelial Carcinoma.
FGFRs are a family of Receptor Tyrosine Kinases, which may be upregulated in a variety of malignancies. The Fibroblast Growth Factor/Fibroblast Growth Factor Receptor (FGF/FGFR) signaling pathway regulates embryogenesis, adult tissue homeostasis, angiogenesis and wound repair, and is also pivotal in cell functions, including proliferation, differentiation, apoptosis and migration. Deregulated FGF/FGFR activations have been associated with developmental disorders and cancer progression. Following binding with a ligand, FGFRs activate downstream signaling pathways such as the Mitogen Activated Protein Kinase (MAPK), Signal Transducer and Activator of Transcription (STAT), the PhosphoInositide-3-Kinase (PI3K)/Akt pathways, and PLC-DAG-PKC pathway. FGFR isoforms have been shown to result in oncogenic FGFR signaling, which in turn promotes tumorigenesis. FGFR3 mutations have been described in approximately 75% of low-grade papillary bladder cancers, and FGFR3 overexpression has been noted in 42% of muscle-invasive bladder cancers. FGFR1 amplification has also been found in 3% of urinary bladder cancers. Patients with FGFR alterations have poor outcomes when treated with available therapies and these alterations occur in 20% of patients with metastatic Urothelial Carcinoma.
BALVERSA® (Erdafitinib) is a once-daily, oral, pan-Fibroblast Growth Factor Receptor (FGFR) Tyrosine Kinase Inhibitor. The approval of BALVERSA® was based on data from a cohort of 87 patients, enrolled in Study BLC2001, which is a multicenter, open-label, single-arm trial. Enrolled patients had locally advanced or metastatic Urothelial Carcinoma that had progressed during, or following at least one prior chemotherapy regimen, and had FGFR genomic alterations such as FGFR3 gene mutations or FGFR2 or FGFR3 gene fusions. Ten percent of patients were chemo naïve, 47% percent of patients had received two or more prior lines of therapy and 80% of patients had visceral metastases. Approximately 97% patients had prior Platinum based therapy and 24% of patients had received anti–PD-1/PD-L1 treatment. The median patient age was 67 years. Patients received BALVERSA® at a starting dose of 8 mg PO once daily. Patients whose serum phosphate levels were below the target of 5.5 mg/dL between days 14 and 17 (41% of the patients) had their dose increased to 9 mg once daily. Treatment was continued until disease progression or unacceptable toxicity. The Primary end point was Objective Response Rate (ORR).
The ORR was 32.2%, with Complete Responses in 2.3% and Partial Responses in 29.9%. Median response duration was 5.4 months. Responding patients included those patients who had previously not responded to anti PD-L1 or PD-1 treatment. The most common adverse reactions were increased serum phosphate, stomatitis, fatigue, increased serum creatinine, diarrhea, onycholysis, increased liver function studies and hyponatremia.
The authors concluded that treatment with BALVERSA® resulted in high Response Rates among patients with chemorefractory metastatic Urothelial Carcinoma with FGFR genomic alterations. BALVERSA® is the first approved personalized treatment, targeting susceptible FGFR genetic alterations, fulfilling an unmet need for these poor prognosis patients. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt). Siefker-Radtke AO, Necchi A, Park SH, et al. J Clin Oncol 36, 2018 (suppl; abstr 4503)
BALVERSA® (Erdafitinib)
The FDA on April 12, 2019 granted accelerated approval to BALVERSA® (Erdafitinib) for patients with locally advanced or metastatic Urothelial Carcinoma, with susceptible FGFR3 or FGFR2 genetic alterations,that has progressed during or following Platinum-containing chemotherapy, including within 12 months of neoadjuvant or adjuvant Platinum-containing chemotherapy. Patients should be selected for therapy based on an FDA-approved companion diagnostic for BALVERSA®. The FDA also simultaneously approved the THERASCREEN® FGFR RGQ RT-PCR Kit, developed by QIAGEN, for use as a companion diagnostic for this therapeutic indication. BALVERSA® is a product of Janssen Pharmaceutical Companies.
KEYTRUDA® (Pembrolizumab)
The FDA on April 11, 2019, approved KEYTRUDA® (Pembrolizumab) for the first-line treatment of patients with Stage III Non-Small Cell Lung Cancer (NSCLC) who are not candidates for surgical resection or definitive chemoradiation, as well as those with metastatic NSCLC. Patients’ tumors must have no EGFR or ALK genomic aberrations and express PD-L1 (Tumor Proportion Score-TPS of 1% or more), as determined by an FDA-approved test. KEYTRUDA® was previously approved as a single agent for the first-line treatment of patients with metastatic NSCLC whose tumors express PD-L1 TPS of 50% or more. KEYTRUDA® is a product of Merck Inc.
2019 NCCN Pancreatic Cancer Guideline Update Draw Attention to Germline Testing and Molecular Profiling
SUMMARY: The American Cancer Society estimates that for 2019, about 56,770 people will be diagnosed with pancreatic cancer and about 45,750 people will die of the disease. Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States and Western Europe. Unfortunately, unlike other malignancies, very little progress has been made, and outcomes for patients with advanced pancreatic cancer has been dismal, with a 5-year survival rate for metastatic pancreatic cancer of approximately 2%. Pancreatic cancer has surpassed breast cancer as the third leading cause of cancer death in the United States and is on track to surpass colorectal cancer, to move to the second leading cause of cancer related deaths in the United States around 2020.
At the 2019 NCCN Annual Conference, three important pancreatic cancer guideline updates were discussed. They included germline testing, molecular analysis of tumors and a new adjuvant chemotherapy option for pancreatic adenocarcinoma.
Germline Testing
Germline testing should be considered for all patients with pancreatic cancer and is especially recommended for those with a personal history of cancer, family history or clinical suspicion of a family history of pancreatic cancer. Approximately 10% of pancreatic cancer cases have a familial component. When hereditary cancer syndrome is suspected in patients with pancreatic cancer, genetic counseling should be considered.
1) Lynch Syndrome (Hereditary Nonpolyposis Colorectal Carcinoma – HNPCC) is a Autosomal Dominant disorder caused by germline mutations in DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6 or PMS2 and most often predisposes to colorectal cancer. Patients with Lynch Syndrome also have a 9-11 fold increase in the risk for pancreatic cancer. Consider testing for MSI and/or MMR for patients with locally advanced or metastatic pancreatic adenocarcinoma.
2) BRCA1/2 mutations have been detected in 4-7% of patients with pancreatic cancer, with a 2-6 fold increase in risk, associated with these mutations. These patients tend to be younger. Among pancreatic cancer patients with Ashkenazi Jewish ancestry, the prevalence of BRCA1/2 mutations is 6-19%, with mutations more common for BRCA2.
3) Mutations in Fanconi Anemia/BRCA pathway genes including PALB, FANCC and FANCG have also been identified as increasing pancreatic cancer risk.
4) Germline mutations in ATM gene has been identified in approximately 4% of individuals with familial pancreatic cancer.
5) Germline mutations in STK11 gene resulting in Peutz-Jeghers syndrome (associated with GI polyps) increases the risk of developing pancreatic cancer 132 fold. In approximately 5% of pancreatic cancers, somatic mutations in STK11 has been noted.
6) Similar to non-hereditary forms of pancreatitis, familial pancreatitis is also associated with increased risk of pancreatic cancer. Those with familial pancreatitis have been noted to have mutations in the PRSS1, SPINK1 and CFTR genes, increasing the risk of developing pancreatic cancer by 26-87 fold.
7) Familial malignant melanoma syndrome, also known as melanoma–pancreatic cancer syndrome or Familial Atypical Multiple Mole Melanoma (FAMMM) syndrome, is associated with a 20-47 fold increased risk of pancreatic cancer. This has been attributed to germline mutation of CDKN2A gene.
Molecular Profiling
Molecular analysis of tumors should be considered for patients with metastatic disease, for treatment guidance
1) In the phase III POLO trial, patients with germline BRCA-mutated metastatic adenocarcinoma of the pancreas, benefited with PARP inhibitor, LYNPARZA® (Olaparib), which when given as frontline maintenance therapy, significantly reduced the risk of disease progression or death, when compared to placebo.
2) Patients with unresectable or metastatic MSI-High or MMR deficient (dMMR) solid tumors who had progressed on prior therapies, have significant responses with KEYTRUDA® (Pembrolizumab), and has been approved by the FDA for this indication.
3) For those patients with PALB2 mutation, Gemcitabine along with Cisplatin is a treatment option.
4) The presence of P16 alterations in resected tumors of patients with pancreatic adenocarcinoma is associated with a worse prognosis and may therefore benefit from adjuvant chemotherapy.
Adjuvant mFOLFIRINOX
In a large phase III multicenter, randomized clinical trial, adjuvant mFOLFIRINOX significantly improved Disease Free Survival, Metastasis Free Survival and Overall Survival, compared to Gemcitabine, after pancreatic cancer resection. The median OS was nearly 20 months longer with a mFOLFIRINOX regimen than with Gemcitabine (54.4 months versus 35 months), representing a 34% reduction in the risk of death with mFOLFIRINOX.
NCCN Guidelines Updates: Tempero MA. Treatment of Pancreatic Cancer. Presented at: 2019 NCCN Annual Conference; March 21-23, 2019; Orlando, FL.
AACR Late-Breaking Research Predicting Response to Anti-PD1/PDL1 Therapy beyond Tumor Mutational Burden
SUMMARY: Immunotherapy with checkpoint inhibitors such as anti-PD1/PDL1 antibodies, is rapidly moving to the forefront of cancer treatment. These agents include PD1 targeted therapies such as KEYTRUDA® (Pembrolizumab), OPDIVO® (Nivolumab) and LIBTAYO® (Cemiplimab-rwlc) and PDL1 targeted therapies such as TECENTRIQ® (Atezolizumab), IMFINZI® (Durvalumab) and BAVENCIO® (Avelumab). Treatment with checkpoint inhibitors given as a single agent or in combination with chemotherapy has resulted in significant survival benefit in a variety of solid tumors, as well as hematologic malignancies. The efficacy of checkpoint inhibitors however varies considerably across different cancer types. Understanding tumors and their microenvironment and identifying the underlying variables that predict response to anti-PD1/PDL1 antibodies, has been challenging.
Tumor Mutational Burden (TMB) has recently emerged as a potential biomarker for immunotherapy with anti PD-1/PDL1 antibodies. TMB can be measured using Next-Generation Sequencing (NGS) and is defined as the number of somatic coding base substitutions and short insertions and deletions (indels), per megabase of genome examined. Several studies have incorporated Tumor Mutational Burden (TMB) as a biomarker, using the validated cutoff of TMB of 10 or more mutations/megabase as High, and less than 10 mutations/megabase, as Low. Drawbacks with TMB include sample consumption, higher attrition rate due to sample quality and quantity, and lack of standardization for the different TMB testing assays, with the definition of High TMB varying across studies from 7.4 or more to 20 mutations/megabase.
The Cancer Genome Atlas (TCGA), a landmark cancer genomics program, is a joint effort between the National Cancer Institute and the National Human Genome Research Institute. This program began in 2006 and has molecularly characterized over 20,000 primary cancers and matched normal samples, across 33 different cancer types. After 12 years and contributions from over 11,000 patients, TCGA has deepened our understanding of the molecular basis of cancer, changed the way cancer patients are managed in the clinic, established a rich genomics data resource for the research community and helped advance health and science technologies.
The authors in this study systematically analyzed Whole Exome Sequencing (WES) and RNA sequencing (RNAseq) data of 10,000 patients from the Cancer Genome Atlas, and the Overall Response Rate (ORR) to anti-PD1/PDL1 therapy of 21 different cancer types obtained from previous clinical trials. The researchers took into consideration more than 30 different variables belonging to three distinct classes: a) those associated with tumor neoantigen landscape (Tumor Mutational Burden-TMB) b) tumor microenvironment and inflammation, and c) the checkpoint inhibitor targets (PD1/PDL1). The performance of each of these variables and their combinations was then evaluated in predicting the ORR to anti-PD1/PDL1 therapy.
It was noted that the most important predictor of response to anti-PD1/PDL1 therapy across cancer types was CD8+ T-cell abundance in the tumor microenvironment, followed by the Tumor Mutational Burden, and a high PD1 gene expression in each cancer type in a fraction of samples. These three top predictors encompassed the three distinct classes considered in this analysis, and their combination was highly predictive of the ORR to anti-PD1/PDL1 therapy, and was able to explain more than 80% of the variance observed across different tumor types.
The authors concluded that in this first systemic evaluation of the different variables associated with PD1/PDL1 therapy response across different tumor types, the three top predictors mentioned above can explain most of the observed cross-cancer response variability. Combining tumor mutational burden, CD8+ T-cell abundance and PD1 mRNA expression accurately predicts response to anti-PD1/PDL1 therapy across cancers. Lee JS and Ruppin E. Presented at: 2019 AACR Annual Meeting; March 29 to April 3, 2019; Atlanta, GA.LB-017/9
