SUMMARY: The FDA on August 16, 2019, approved INREBIC® (Fedratinib) for adults with Intermediate-2 or High-risk Primary or Secondary (post-Polycythemia Vera or post-Essential Thrombocythemia) Myelofibrosis (MF).
Myelofibrosis is a MyeloProliferative Neoplasm (MPN) characterized by ineffective hematopoiesis, progressive fibrosis of the bone marrow and potential for leukemic transformation. This stem cell disorder is Philadelphia Chromosome negative and manifestations include anemia, splenomegaly and related symptoms such as abdominal distension and discomfort with early satiety. Cytokine driven debilitating symptoms such as fatigue, fever, night sweats, weight loss, pruritus and bone or muscle pain can further impact an individual’s quality of life. Myelofibrosis can be Primary (PMF) or Secondary to Polycythemia Vera (PV) or Essential Thrombocythemia (ET).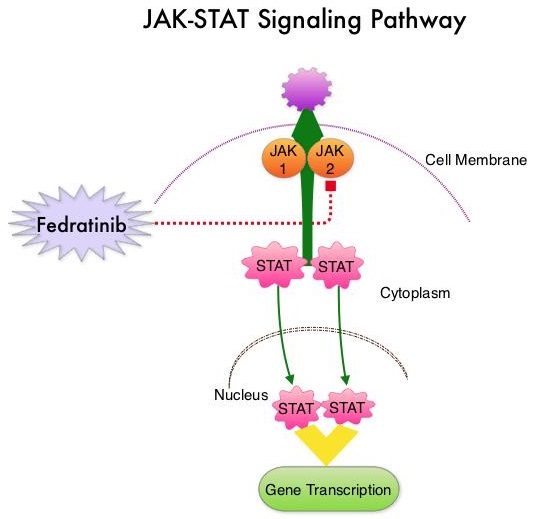 The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling. Currently, JAKAFI® (Ruxolitinib) is the only drug that has been approved by the FDA for Myelofibrosis.
The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling. Currently, JAKAFI® (Ruxolitinib) is the only drug that has been approved by the FDA for Myelofibrosis.
INREBIC® is a highly selective JAK2 inhibitor and is active against a broader family of kinases and kinase mutants. This is unlike JAKAFI® which is a JAK1/2 inhibitor.  The approval of INREBIC®was based on findings from the Phase III JAKARTA-2 study, which evaluated INREBIC® in patients with Primary or Secondary Myelofibrosis. In this double-blind, randomized, placebo-controlled trial, 289 patients with Intermediate-2 or High-risk MF, post-Polycythemia Vera MF, or post-Essential Thrombocythemia MF with splenomegaly, were randomized to receive either INREBIC® 500 mg (N=97), 400 mg (N=96) or placebo (N=96), once daily for at least 6 consecutive 4-week cycles. The Primary endpoint was the proportion of patients achieving 35% or more reduction in spleen volume from baseline at the end of cycle 6 measured by MRI or CT, with a follow up scan 4 weeks later. Secondary end point was symptom response (50% or more reduction in total symptom score, assessed using the modified Myelofibrosis Symptom Assessment Form).
The approval of INREBIC®was based on findings from the Phase III JAKARTA-2 study, which evaluated INREBIC® in patients with Primary or Secondary Myelofibrosis. In this double-blind, randomized, placebo-controlled trial, 289 patients with Intermediate-2 or High-risk MF, post-Polycythemia Vera MF, or post-Essential Thrombocythemia MF with splenomegaly, were randomized to receive either INREBIC® 500 mg (N=97), 400 mg (N=96) or placebo (N=96), once daily for at least 6 consecutive 4-week cycles. The Primary endpoint was the proportion of patients achieving 35% or more reduction in spleen volume from baseline at the end of cycle 6 measured by MRI or CT, with a follow up scan 4 weeks later. Secondary end point was symptom response (50% or more reduction in total symptom score, assessed using the modified Myelofibrosis Symptom Assessment Form).
The Primary end point was achieved by 37% of the patients receiving INREBIC® at the 400 mg dose compared with 1% of patients who were in the placebo group (P <0.0001). The median duration of spleen response was 18.2 months for the INREBIC® 400 mg group. Further, 40% of patients who received INREBIC® at the 400 mg dose, experienced a 50% or more reduction in Myelofibrosis-related symptoms, whereas only 9% of patients receiving placebo experienced a decrease in these symptoms (P<0.0001). The most common adverse reactions seen in 20% or more patients who received INREBIC® were diarrhea, nausea, anemia, and vomiting.
In conclusion, INREBIC® therapy significantly reduced splenomegaly and symptom burden in patients with Myelofibrosis. This FDA approval represents an important milestone for patients with Myelofibrosis and clinicians now have a new treatment option for these patients, including those previously treated with JAKAFI®. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fedratinib-myelofibrosis
