The FDA on November 8, 2019 approved REBLOZYL® for treatment of anemia in adult patients with beta Thalassemia who require regular red blood cell transfusions. REBLOZYL® is a product of Celgene Corp.
Month: November 2019
BRUKINSA® (Zanubrutinib)
The FDA on November 14, 2019 granted accelerated approval to BRUKINSA® for adult patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. BRUKINSA® is a product of BeiGene, Ltd.
ADAKVEO® (Crizanlizumab-tmca)
The FDA on November 15, 2019 approved ADAKVEO® to reduce the frequency of Vaso-Occlusive Crises (VOCs) in adults and pediatric patients aged 16 years and older with Sickle Cell disease. ADAKVEO® is a product of Novartis Pharmaceuticals Corporation.
GIVLAARI® (Givosiran)
The FDA on November 20, 2019 approved GIVLAARI® for adults with Acute Hepatic Porphyria (AHP). GIVLAARI® is a product of Alnylam Pharmaceuticals, Inc.
CALQUENCE® (Acalabrutinib)
The FDA on November 21, 2019 approved CALQUENCE® for adults with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). CALQUENCE® is a product of AstraZeneca.
OXBRYTA® (Voxelotor)
The FDA on November 25, 2019 granted accelerated approval to OXBRYTA® for adults and pediatric patients 12 years of age and older with Sickle Cell disease. OXBRYTA® is a product of Global Blood Therapeutics.
First Line Combination of TECENTRIQ® and AVASTIN® Improves Survival in Hepatocellular Carcinoma
SUMMARY: The American Cancer Society estimates that for 2019, about 42,030 new cases of primary liver cancer will be diagnosed in the US and 31,780 patients will die of their disease. Liver cancer is seen more often in men than in women and the incidence has more than tripled since 1980. This increase has been attributed to the higher rate of Hepatitis C Virus (HCV) infection among baby boomers (born between 1945 through 1965). Obesity and Type II diabetes have also likely contributed to the trend. Other risk factors include alcohol, which increases liver cancer risk by about 10% per drink per day, and tobacco use, which increases liver cancer risk by approximately 50%. HepatoCellular Carcinoma (HCC) is the second most common cause of cancer-related deaths worldwide and majority of patients typically presents at an advanced stage. The prognosis for unresectable HCC remains poor and one year survival rate is less than 50% following diagnosis. NEXAVAR® was approved by the FDA in 2007 for the first line treatment of unresectable HepatoCellular Carcinoma (HCC) and the median Overall Survival was 10.7 months in the NEXAVAR® group and 7.9 months in the placebo group.
TECENTRIQ® (Atezolizumab) is an anti PD-L1 monoclonal antibody, designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating immune cells, thereby blocking its interactions with PD-1 and B7.1 receptors, and thus enabling the activation of T cells. AVASTIN® (Bevacizumab) is a recombinant humanized monoclonal IgG1 antibody that binds VEGF (Vascular Endothelial Growth Factor) and prevents the interaction of VEGF to its receptors (Flt-1 and KDR) on the surface of endothelial cells, thereby preventing endothelial cell proliferation and new blood vessel formation. The use of TECENTRIQ® in combination with AVASTIN® has a strong scientific rationale, as this combination can potentially enhance the immune system to combat a broad range of malignancies. AVASTIN® in addition to its established anti-angiogenic properties can further enhance TECENTRIQ®’s ability to restore anti-cancer immunity, by inhibiting VEGF-related immunosuppression, promoting T-cell tumor infiltration and enabling priming and activation of T-cell responses against tumor antigens.
IMbrave150 is a global, open-label, multicenter, randomized, Phase III study in which a combination of TECENTRIQ® and AVASTIN® was compared with standard-of-care NEXAVAR®, in patients with previously untreated locally advanced or metastatic HCC. Patients were randomized 2:1 to receive TECENTRIQ® 1200 mg IV on day 1 along with AVASTIN® 15 mg/kg on day 1 of each 21-day cycle (N=336) or NEXAVAR® 400 mg orally twice daily, each day of the 21-day cycle (N=165). Treatment was continued until disease progression or unacceptable toxicity. The treatment groups were well balanced and enrolled patients had an ECOG performance status of 0 or 1, Child-Pugh Class A disease, and adequate hematologic and end-organ function. The two co-Primary endpoints were Overall Survival (OS) and Progression Free Survival (PFS). The key Secondary endpoints included Overall Response Rate (ORR), Time To Progression (TTP) and Duration of Response (DOR), as well as Patient-Reported Outcomes (PROs), Safety and Pharmacokinetics.
With a median follow up of 8.6 months, the OS was not yet reached in the TECENTRIQ® and AVASTIN® combination group compared with 13.2 months in the NEXAVAR® group (HR=0.58; P=0.0006). The median PFS was 6.8 months versus 4.3 months respectively (HR=0.59; P<0.0001). The ORR was 27% versus 12% (P<0.0001) based on the Independent Review Facility RECIST 1.1 criteria, in favor of the combination regimen. This benefit was seen across clinical subgroups and the combination regimen delayed deterioration of Quality of Life compared with NEXAVAR®. Grade 3 and 4 Adverse Events were similar and occurred in 57% and 55% of the combination and control arms, respectively.
It was concluded that a combination of TECENTRIQ® and AVASTIN® demonstrated statistically significant and clinically meaningful improvement in both Overall Survival and Progression Free Survival compared with NEXAVAR®, in treatment naïve patients with unresectable Hepatocelluar Carcinoma. The authors added that this is the first study in 11 years to show an improvement in Overall Survival with a new first line treatment option, compared to NEXAVAR®, and has the potential to be a practice changing treatment in Hepatocellular Carcinoma. IMbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Cheng A-L, Qin S, Ikeda M, et al. Annals of Oncology, Volume 30, 2019 Supplement 9. LBA3
FDA Approves CALQUENCE® for Chronic Lymphocytic Leukemia
SUMMARY: The FDA on November 21, 2019, approved CALQUENCE® (Acalabrutinib), for adults with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). The American Cancer Society estimates that for 2019, about 20,720 new cases of Chronic Lymphocytic Leukemia (CLL) will be diagnosed in the US and 3,930 patients will die of the disease. CLL accounts for about 25% of the new cases of leukemia and the average age at the time of diagnosis is around 71 years. B-cell CLL is the most common type of leukemia in adults, accounting for about 11% of all hematologic malignancies.
Bruton's Tyrosine Kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. CALQUENCE® is a highly selective, oral, covalent irreversible Bruton Tyrosine Kinase (BTK) inhibitor with minimal activity against other kinases. CALQUENCE® inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. The present FDA approval was based on two randomized, actively controlled trials, ELEVATE-TN and ASCEND in patients with CLL.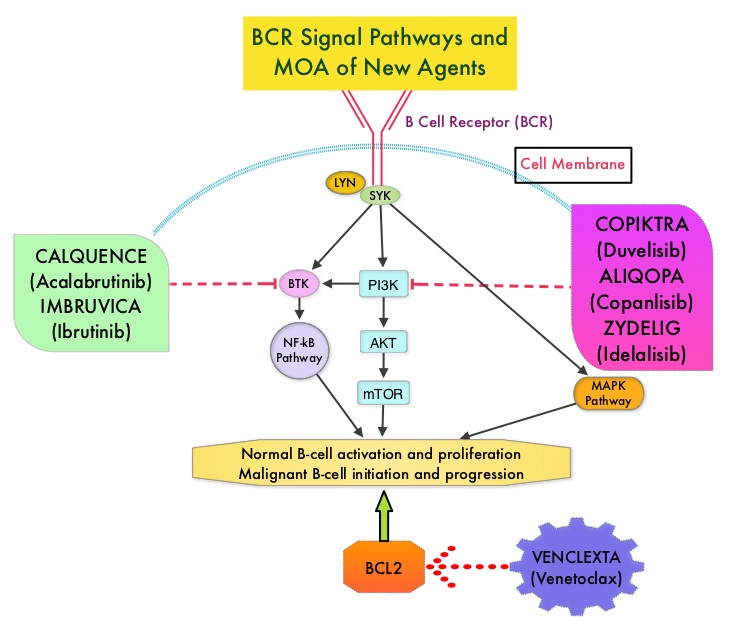
ELEVATE-TN is a randomized, multicentre, open-label Phase III trial which evaluated the safety and efficacy of CALQUENCE® alone or in combination with GAZYVA® (Obinutuzumab) versus Chlorambucil in combination with GAZYVA®, in previously-untreated patients with CLL. GAZYVA® is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody. In this trial, 535 patients were randomized 1:1:1 to receive CALQUENCE® monotherapy 100 mg twice daily orally continuously (N=179) or in combination with GAZYVA® (N=179), or Chlorambucil 0.5 mg/kg on Days 1 and 15 of each 28-day cycle for 6 cycles along with GAZYVA® (N=177). The dose of GAZYVA® was 100 mg on day 1 and 900 mg on day 2 of Cycle 1, 1000 mg on day 8 and 15 of Cycle 1, and 1000 mg on day 1 of Cycles 2–6, of each 28 day cycle. Pts were stratified by del(17p) status, ECOG status (1 or less versus 2), and geographic region. The median patient age was 70 years and 69% had high risk and 12% had very high risk CLL IPI scores. The Primary endpoint was PFS in the CALQUENCE® plus GAZYVA® group compared to the Chlorambucil and GAZYVA® group, assessed by an independent review committee (IRC). A key secondary endpoint was PFS in the CALQUENCE® monotherapy arm, compared to the chlorambucil and GAZYVA® arm. Other secondary endpoints included Objective Response Rate, time to next treatment and Overall Survival.
At a median follow-up of 28 months, the combination of CALQUENCE® plus GAZYVA® significantly prolonged PFS compared to Chlorambucil plus GAZYVA® (median Not Reached versus 22.6 months; HR 0.10, P<0.0001), reducing the risk of progression or death by 90%. CALQUENCE® monotherapy also significantly prolonged PFS compared to Chlorambucil plus GAZYVA® (HR=0.20; P<0.0001). PFS improvement with CALQUENCE® plus GAZYVA® as well as CALQUENCE® monotherapy was consistent across all subgroups examined including del(17p). The median OS was not reached in any treatment group. The ORR was higher with CALQUENCE® plus GAZYVA® versus Chlorambucil plus GAZYVA® (94% versus 79%; P<0.0001). The ORR with CALQUENCE® monotherapy was 85%. Complete Response rates were higher with CALQUENCE® plus GAZYVA® versus Chlorambucil plus GAZYVA® (13% versus 5%).
ASCEND is a global, randomized, multicentre, open-label Phase III trial which evaluated the efficacy and safety of CALQUENCE® versus physician’s choice of Rituximab/ZYDELIG® (Idelalisib) or Rituximab/Bendamustine combination in patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). This study included 310 eligible patients who were randomly assigned 1:1 to receive CALQUENCE® 100 mg orally twice daily until disease progression versus Rituximab/ ZYDELIG® combination (ZYDELIG® 150 mg orally twice daily in combination with up to eight IV infusions of Rituximab 375 or 500 mg/m2) or Rituximab/Bendamustine combination (Bendamustine 70 mg/m2 IV on day 1 and 2 of each cycle in combination with Rituximab 375 or 500 mg/m2 IV on day 1 of each 28-day cycle for up to six cycles). The median age was 67 years and patients were stratified by del(17p) status, ECOG performance scale and prior lines of therapy. The Primary endpoint was Progression Free Survival as assessed by Independent Review Committee (IRC) and Secondary end points included Overall Response Rate and Duration of Response, as well as Overall Survival. Patients with confirmed disease progression on Rituximab/ ZYDELIG® or Rituximab/Bendamustine, were allowed to cross over to receive CALQUENCE®.
At a median follow-up of 16.1 months, there was a statistically significant and clinically meaningful improvement in PFS with CALQUENCE® monotherapy compared to a combination regimen of Rituximab plus physician’s choice of ZYDELIG® or Bendamustine. The PFS with CALQUENCE® monotherapy was not reached versus 16.5 months with the comparators (HR= 0.31, P<0.0001). This represented a 69% reduction in risk of progression or death. Progression Free Survival was improved with CALQUENCE® monotherapy across subgroups including del(17p), TP53 mutation, and Rai stage. There was however no significant difference in the Overall Response Rates among the treatment groups and 23% of the patients randomly assigned to Rituximab/ ZYDELIG® or Rituximab/Bendamustine combinations crossed over to receive subsequent treatment with CALQUENCE®. The most common side effects of CALQUENCE® were anemia, neutropenia, upper respiratory tract infection, thrombocytopenia, headache, diarrhea, and musculoskeletal pain.
In conclusion, CALQUENCE® monotherapy as well as in combination with GAZYVA®, significantly improved Progression Free Survival in Relapsed/Refractory CLL, as well as treatment naïve patients with CLL, respectively.
ELEVATE TN: Phase 3 Study of Acalabrutinib Combined with Obinutuzumab (O) or Alone Vs O Plus Chlorambucil (Clb) in Patients (Pts) with Treatment-Naive Chronic Lymphocytic Leukemia (CLL). Sharman JP, Banerji V, Fogliatto LM, et al. Blood. 2019 Nov 13;134(Supplement_1):31.
ASCEND phase 3 study of acalabrutinib vs investigator’s choice of rituximab plus idelasib (IDR) or bendamustine (BR) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Ghia P, Pluta A, Wach M, et al. Presented at: 2019 European Hematology Association Congress; June 13-16, 2019; Amsterdam, Netherlands. Abstract LB2606.
Higher Levels of IGF-1 and Free Testosterone Increase the Risk of Prostate Cancer
SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer, and 1 in 9 men will be diagnosed with Prostate cancer during their lifetime. It is estimated that in the United States, about 174,650 new cases of Prostate cancer will be diagnosed in 2019 and 31,620 men will die of the disease. The etiology of Prostate cancer remains unclear, and North American Blacks are at the highest risk whereas risk is lowest in Asians, with the risk among Caucasians somewhere in between.
The Insulin-like Growth Factor (IGF) system has been reported to regulate normal and malignant cell growth, proliferation and differentiation, tissue homeostasis and cellular metabolism, and its relevance in carcinogenesis has been well established. The role of IGF system in Prostate cancer was initially recognized from epidemiological studies which showed that higher serum IGF-1 concentrations correlated with an increased risk of Prostate cancer. Testosterone in the circulation is bound primarily to Sex Hormone Binding Globulin (SHBG) while the unbound, or free testosterone, is the most bioavailable and active form.
The authors in this large prospective study investigated the association of circulating levels of IGF-I, free (biologically active testosterone) and total testosterone, and Sex Hormone Binding Globulin (SHBG) with Prostate cancer incidence and mortality, in a large cohort of patients. This study included 200,452 male participants who were free of cancer when they were enrolled in the study, and were not taking any hormone therapy. Baseline blood samples were obtained from these men and tested for levels of total testosterone and IGF-1. Free testosterone level was calculated from measured total testosterone and binding protein concentrations.
After a mean follow up of 6.9 years, 5412 men were diagnosed with Prostate cancer and 296 had died from the disease. It was noted that higher levels of circulating IGF-I was associated with an elevated risk of Prostate cancer diagnosis, as well as Prostate cancer mortality. Higher free testosterone level was associated with an elevated risk of incident Prostate cancer, whereas higher SHBG was associated with a lower risk, but neither was associated with Prostate cancer mortality. Total testosterone levels were not associated with Prostate cancer incidence or mortality. For every 5 nanomoles increase in the concentration of IGF-1 per liter of blood (5 nmol/L), men were 9% more likely to develop Prostate cancer. For every 50 picomoles increase of free testosterone per liter of blood (50 pmol/L), there was a 10% increase in Prostate cancer risk. The researchers added that their findings correspond to a 25% greater risk of Prostate cancer in men who have the highest levels of IGF-1, compared to those with the lowest, and men with the highest free testosterone levels have a 18% greater risk of Prostate cancer, compared to those with the lowest levels.
It was concluded from this study that circulating levels of IGF-I and free testosterone play a role in the development of Prostate cancer and these two hormones could be one of the several mechanisms that links diet, lifestyle, and body size with the risk of Prostate cancer, taking us one step closer to Prostate cancer prevention. Serum hormones and prostate cancer incidence and mortality in UK Biobank. Travis R, Watts E, Fensom G, et al. Presented at the 2019 NCRI Cancer Conference. Abstract#2904
FDA Approves ADAKVEO®, A New Targeted Therapy for Sickle Cell Disease
SUMMARY: The FDA on November 15, 2019 approved ADAKVEO® (Crizanlizumab-tmca), a treatment to reduce the frequency of vaso-occlusive crisis, for patients age 16 years and older. Vaso-occlusive crisis is a common and painful complication of Sickle Cell Disease (SCD), that occurs when blood circulation is obstructed by sickled red blood cells. Sickle Cell Disease or Sickle Cell anemia is an Autosomal Recessive disorder and affects approximately 100,000 Americans. It is estimated that it affects 1 out of every 365 African-American births and 1 out of every 16,300 Hispanic-American births. The average life expectancy for patients with Sickle Cell Disease in the United States is approximately 40-60 years.
HbSS disease or Sickle Cell anemia is the most common Sickle Cell Disease genotype and is associated with the most severe manifestations. HbSS disease is caused by a mutation substituting thymine for adenine in the sixth codon of the beta-globin chain gene. This in turn affects the hemoglobin’s ability to carry oxygen and causes it to polymerize. This results in decreased solubility thereby distorting the shape of the red blood cells, increasing their rigidity and resulting in red blood cells that are sickle shaped rather than biconcave. These sickle shaped red blood cells limit oxygen delivery to the tissues by restricting the flow in blood vessels, leading to severe pain and organ damage (vaso-occlusive crises). Oxidative stress is an important contributing factor to hemoglobin polymerization with polymer formation occurring only in the deoxy state. HbS/b-0 thalassemia (double heterozygote for HbS and b-0 thalassemia) is clinically indistinguishable from HbSS disease.
P-Selectin is a Cell Adhesion Molecule (CAM) expressed on the surfaces of activated vascular endothelial cells and platelets. In unactivated endothelial cells and platelets, it is stored in Weibel-Palade bodies and alpha granules respectively. P-Selectin is released from endothelial cells and platelets when activated by inflammation or trauma and mediates the binding of erythrocytes and leukocytes to the vessel wall. In patients with Sickle Cell Disease (SCD), adherent masses of sickled red cells and leukocytes contribute to vaso-occlusive pain crises. ADAKVEO® is a first-in-class humanized anti-P-Selectin antibody, and the SUSTAIN study evaluated the safety and efficacy of ADAKVEO® on the frequency of Sickle Cell-related Pain Crises (SCPC) in Sickle Cell Disease patients.
The present FDA approval of ADAKVEO® was based on SUSTAIN, a multicenter, randomized, placebo controlled, double-blind clinical trial in which 198 Sickle Cell Disease patients with a history of vaso-occlusive crisis, were randomly assigned to receive ADAKVEO® at doses of 5 mg/kg, 2.5 mg/kg, or placebo, administered intravenously, 14 times over 52 weeks. Treatment groups were well balanced and patients receiving Hydroxyurea or Erythropoietin were included, if prescribed for the preceding 6 months and dose was stable for at least 3 months. The Primary end point was Sickle Cell-related Pain Crises (SCPC), defined as acute sickle cell-related pain that resulted in a visit to a medical facility and required a parenteral or oral narcotic or parenteral NSAID. Acute Chest Syndrome (ACS), priapism, hepatic and splenic sequestration were also included in this definition.
Treatment with ADAKVEO® experienced fewer health care visits for vaso-occlusive crisis annually and significantly lowered the median annual rate of vaso-occlusive crisis by 45% from 2.98 visits to 1.63 visits, compared with placebo. Reductions in the frequency of vaso-occlusive crisis were observed among patients regardless of Sickle Cell Disease genotype and/or Hydroxyurea use. More than one third (36%) of patients who received ADAKVEO® did not experience vaso-occlusive crisis during the study, compared with 17% of placebo-treated patients. ADAKVEO® delayed the time that patients first experienced vaso-occlusive crisis after starting treatment from a median of 1.4 months to 4.1 months. Common side effects associated with ADAKVEO® included back pain, nausea, fever and arthralgia. Patients should be monitored for infusion-related reactions and treatment should be discontinued for severe reactions. Patients should also be monitored for interference with automated platelet counts or platelet clumping and it is advised that CBC be performed using citrate tubes to avoid platelet activation.
It was concluded that ADAKVEO® significantly reduced Sickle Cell-related Pain Crises (SCPC) and increased the time to first and second SCPC. The authors added that chronic inhibition of P-Selectin with once a month IV dosing of ADAKVEO® represents a novel and potentially new disease-modifying, prophylactic treatment option for patients with Sickle Cell Disease. SUSTAIN: A Multicenter, Randomized, Placebo-Controlled, Double-Blind, 12-Month Study to Assess Safety and Efficacy of SelG1 with or without Hydroxyurea Therapy in Sickle Cell Disease Patients with Sickle Cell-Related Pain Crises. Ataga KI, Kutlar A, Kanter J, et al. N Engl J Med 2017; 376:429-439
