SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their life time. Approximately, 246,660 new cases of invasive breast cancer will be diagnosed in 2016 and 40,450 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive (Estrogen Receptor/Progesterone Receptor positive) and this is a predictor of response to endocrine therapy. These patients are often treated with anti-estrogen therapy as first line treatment. In premenopausal woman, the ovary is the main source of estrogen production, whereas in postmenopausal women, the primary source of estrogen is the Aromatase enzyme mediated conversion of androstenedione and testosterone to estrone and estradiol, in extragonadal/peripheral tissues. NOLVADEX® (Tamoxifen) is a nonsteroidal Selective Estrogen Receptor Modulator (SERM) and works mainly by binding to the Estrogen Receptor and thus blocks the proliferative actions of estrogen on the mammary tissue. ARIMIDEX® (Anastrozole), FEMARA® (Letrozole) and AROMASIN® (Exemestane) are Aromatase Inhibitors (AIs) that binds to the Aromatase enzyme and inhibit the conversion of androgens to estrogens in the extra-gonadal tissues. The use of Aromatase Inhibitors (AIs) has been long associated with musculoskeletal symptoms, as well as accelerated bone loss, leading to a decrease in Bone Mineral Density (BMD). Approximately 25% of the patients on AIs are non-compliant during the first year of therapy and this has been attributed to musculoskeletal symptoms. Increased risk of Carpal Tunnel Syndrome (CTS) has also been reported with AIs.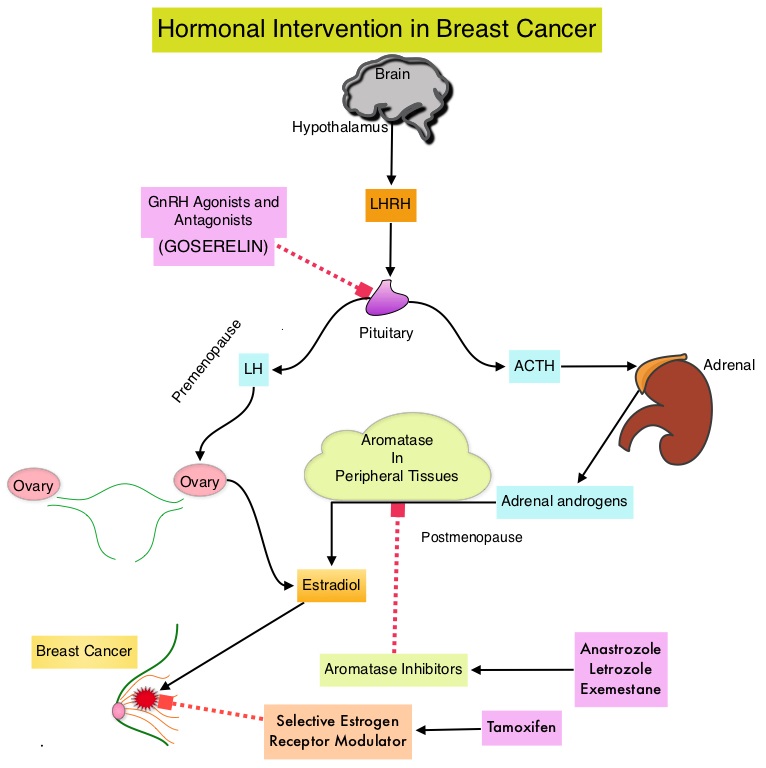
Carpal tunnel syndrome (CTS), also called median nerve compression, is the most common entrapment neuropathy and results from compression of the median nerve, as it runs from the forearm into the palm of the hand, through the carpal tunnel. The carpal tunnel is a narrow and rigid passage at the base of the hand and houses the median nerve and tendons. In most cases, CTS is due to a congenital predisposition, with the carpal tunnel being simply smaller in some individuals than others. Secondary CTS is caused by any condition that further narrows this osteofibrous passage such as arthritis, acromegaly and mechanical problems in the wrist joint or effects the contents of this passage such as tenosynovitis, synovial hypertrophy, hypothyroidism, fluid retention during pregnancy or menopause, or the development of a cyst or tumor in the passage. Bilateral oophorectomy and use of the combined oral contraceptive have also been identified as risk factors for CTS. Patients often experience nocturnal paraesthesias in median nerve distribution (thumb, index and middle fingers), such as burning sensation, tingling, or heaviness, with the pain radiating to the forearm or elbow. It has been postulated that estrogen has antinociceptive properties and estrogen deprivation with AIs decreases the threshold for pain stimuli. Estrogen deprivation may also impact the metabolism of transverse carpal ligament on which estrogen and progesterone receptors are expressed and lack of estrogen may additionally result in morphological changes in the contents of carpal tunnel including, thickening of the tendon sheaths and fluid accumulation. This may directly induce CTS.
To address the risk factors and incidence of CTS in women taking AIs, the authors conducted an exploratory analysis of the International Breast Cancer Intervention Study II, a double-blind randomized clinical trial, in which women at increased risk of breast cancer were randomly assigned to receive ARIMIDEX® or placebo for 5 years. In this study, a total of 3,864 women were randomly assigned to receive either ARIMIDEX® (N=1920) or placebo (N=1944). The median age was 60 years and majority of the women (69%) had a BMI of greater than 25 kg/m2.
After a median follow up of 6.4 years, 96 patients had symptoms of Carpal Tunnel Syndrome (CTS). Patients receiving ARIMIDEX® were more likely to have CTS related symptoms than those receiving placebo (3.4% versus 1.6%; P<0.001). Eight of the 10 participants reported as having severe CTS were taking ARIMIDEX® (P =0.08). Eighteen women (0.9%) in the ARIMIDEX® group required surgical intervention for CTS compared to six women (0.3%) in the placebo group and this was significantly different (P=0.018). Participants experiencing CTS symptoms did so early in the course of treatment and only 6 women discontinued the allocated treatment. In addition to taking AIs, high Body Mass Index and prior complaints of musculoskeletal symptoms, were the only other risk factors for developing CTS.
The authors concluded that the use of ARIMIDEX® was associated with a higher incidence of Carpal Tunnel Syndrome (CTS), although few participants required surgery. Given the association between CTS and other musculoskeletal symptoms induced by AIs (Aromatase Inhibitors), the authors suggested that these findings induced by AIs, may share the same pathobiology. Anastrozole-Induced Carpal Tunnel Syndrome: Results from the International Breast Cancer Intervention Study II Prevention Trial. Spagnolo F, Sestak I, Howell A, et al. J Clin Oncol 2016;34:139-143

