SUMMARY: Selenium and Vitamin E Cancer Prevention Trial (SELECT), is a multicenter, randomized, placebo-controlled trial, conducted by the SWOG cooperative group, that involved more than 35,000 men. Participants were randomized to receive either, a) Selenium and Vitamin E, b) Selenium and a placebo, c) Vitamin E and a placebo or d) Two placebos. The purpose of this trial was to determine if high dose vitamin E (400 IU/day) and/or Selenium (200 mcg/day) supplements could decrease the incidence of prostate cancer. The level/concentration of Selenium in participants toenail clippings was measured at the time of study participation and the goal was to also determine whether Selenium supplements would benefit the subset of participants with low Selenium levels at baseline. Both Vitamin E and Selenium are antioxidants and Vitamin E rich foods include vegetables, vegetable oils, nuts, and egg yolks whereas Selenium a nonmetallic trace element is found in rice, wheat, seafood, meat, and Brazil nuts. 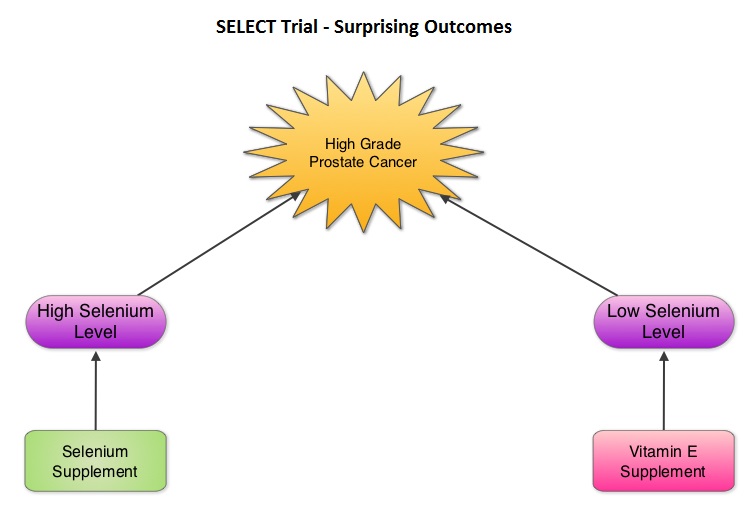 The SELECT trial, which began in 2001, was stopped early in 2008, as Selenium and Vitamin E, taken alone or together for an average of five and a half years did not decrease the incidence of prostate cancer. In 2011, an update on the SELECT trial data suggested that men who were randomized to the vitamin E alone had a 17 percent increased risk of prostate cancer compared to those men taking placebo. The authors in this case–cohort study continued follow up of the SELECT trial participants and with the Selenium levels data from toenail clippings, compared the effect of Selenium and Vitamin E, taken either alone or together, on the risk of prostate cancer, among 1739 men who were diagnosed with prostate cancer, of whom 489 participants developed high-grade prostate cancer. The control group for comparison was a random sample of 3117 men without prostate cancer and they were matched to the cases by race and age. It was noted that an individual’s baseline Selenium level, in the absence of supplementation, was not associated with prostate cancer risk. However, in men who had high baseline Selenium levels, Selenium supplements almost doubled (91%) the risk of high grade prostate cancer (P=0.007). Conversely, Vitamin E supplements had no effect among men with high baseline Selenium levels but doubled the risk of high grade prostate cancer among men with low baseline Selenium levels. Frankel et al. in an accompanying editorial point out that the dose of Vitamin E in the SELECT trial was significantly higher (400 IU/day) than the dose that was selected in the Alpha-Tocopherol Beta Carotene (ATBC) Cancer Prevention trial (50 IU/day), a study that was designed to test Vitamin E and beta carotene for lung cancer prevention in smokers. In the ATBC trial, a decrease in the incidence of prostate cancer incidence was observed, although this was a secondary finding and this study was not designed to determine prostate cancer risk. They comment that high doses of Vitamin E (Alpha-Tocopherol), suppresses the more potentially beneficial serum Gamma-Tocopherol which is the prevalent dietary form of Vitamin E in the United States. Selenium deficiency in the U.S. is not common and any benefit with Selenium supplements can only be seen in those who are Selenium deficient and high doses may be detrimental. The authors concluded that in the SELECT trial, the combination of both Vitamin E and Selenium did not reduce the risk of prostate cancer or any other cancer or heart disease and was in fact harmful for a significant number of individuals. Therefore, men 55 years of age or more should avoid Vitamin E or Selenium supplements at doses that exceed the recommended dietary intake. Kristal AR, Darke AK, Morris JS, et al. J Natl Cancer Inst; First published online 22 February 2014, doi: 10.1093/jnci/djt456
The SELECT trial, which began in 2001, was stopped early in 2008, as Selenium and Vitamin E, taken alone or together for an average of five and a half years did not decrease the incidence of prostate cancer. In 2011, an update on the SELECT trial data suggested that men who were randomized to the vitamin E alone had a 17 percent increased risk of prostate cancer compared to those men taking placebo. The authors in this case–cohort study continued follow up of the SELECT trial participants and with the Selenium levels data from toenail clippings, compared the effect of Selenium and Vitamin E, taken either alone or together, on the risk of prostate cancer, among 1739 men who were diagnosed with prostate cancer, of whom 489 participants developed high-grade prostate cancer. The control group for comparison was a random sample of 3117 men without prostate cancer and they were matched to the cases by race and age. It was noted that an individual’s baseline Selenium level, in the absence of supplementation, was not associated with prostate cancer risk. However, in men who had high baseline Selenium levels, Selenium supplements almost doubled (91%) the risk of high grade prostate cancer (P=0.007). Conversely, Vitamin E supplements had no effect among men with high baseline Selenium levels but doubled the risk of high grade prostate cancer among men with low baseline Selenium levels. Frankel et al. in an accompanying editorial point out that the dose of Vitamin E in the SELECT trial was significantly higher (400 IU/day) than the dose that was selected in the Alpha-Tocopherol Beta Carotene (ATBC) Cancer Prevention trial (50 IU/day), a study that was designed to test Vitamin E and beta carotene for lung cancer prevention in smokers. In the ATBC trial, a decrease in the incidence of prostate cancer incidence was observed, although this was a secondary finding and this study was not designed to determine prostate cancer risk. They comment that high doses of Vitamin E (Alpha-Tocopherol), suppresses the more potentially beneficial serum Gamma-Tocopherol which is the prevalent dietary form of Vitamin E in the United States. Selenium deficiency in the U.S. is not common and any benefit with Selenium supplements can only be seen in those who are Selenium deficient and high doses may be detrimental. The authors concluded that in the SELECT trial, the combination of both Vitamin E and Selenium did not reduce the risk of prostate cancer or any other cancer or heart disease and was in fact harmful for a significant number of individuals. Therefore, men 55 years of age or more should avoid Vitamin E or Selenium supplements at doses that exceed the recommended dietary intake. Kristal AR, Darke AK, Morris JS, et al. J Natl Cancer Inst; First published online 22 February 2014, doi: 10.1093/jnci/djt456

