SUMMARY: It is estimated that in the United States approximately 13,000 people are diagnosed with MyeloDysplastic Syndromes (MDS) each year. MyeloDysplastic Syndromes are a heterogenous group of stem cell disorders characterized by marrow failure resulting in cytopenias with associated cytogenetic abnormalities, and abnormal cellular maturation with morphologic changes in clonal cells. Majority of the individuals diagnosed with MDS are aged 65 years and older and die as a result of infection and/or bleeding consequent to bone marrow failure. About a third of patients with MDS develop Acute Myeloid Leukemia (AML). Patients with low-risk MDS have an indolent disease course with a median survival of about 6 years with no therapeutic intervention. Patients with intermediate and higher-risk disease however have a shorter median survival even with treatment, with approximately a third of the patients progressing to AML within 3 years.
Management of patients with MDS includes supportive care with Erythropoiesis Stimulating Agents (ESA), hypomethylating agents such as VIDAZA® (Azacitidine) and DACOGEN® (Decitabine), immunomodulatory agents such as REVLIMID® (Lenalidomide), and immunosuppressive agents such as AntiThymocyte Globulin (ATG) and Cyclosporine. Symptomatic patients with MDS are often treated with either VIDAZA® or DACOGEN® as these agents have been shown to improve survival in higher-risk MDS patients. It has remained unclear however, if one is better than the other. 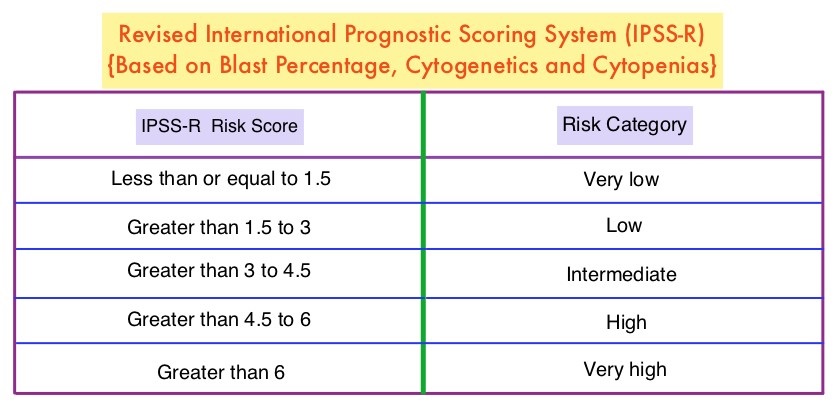
To address this question the authors conducted a phase II study, in which 113 patients with low (36%), intermediate (30%), and high (20%) – risk MDS, as determined by the Revised International Prognostic Scoring System (IPSS-R), were randomly assigned to receive either VIDAZA® 75 mg/m2 IV/SC daily (N=40) or DACOGEN® 20 mg/m2 IV daily (N=73), for 3 consecutive days, with the cycle repeated every 28 days. Patients received a median of 9 cycles. The primary endpoint was Overall Response Rate (ORR).
It was noted that the ORR was 70% and 49% for patients treated with DACOGEN® and VIDAZA® respectively (P=0.03). Cytogenetic response rates were 61% and 25% respectively (P=0.02). Thirty-two percent (32%) of patients treated with DACOGEN® became transfusion independent compared with 16% of patients treated with VIDAZA® Among patients with 5% or more bone marrow blasts, all responded to DACOGEN® whereas only 36% responded to VIDAZA® (P<0.001). With a median follow up of 20 months, the median Event Free Survival for patients treated with DACOGEN® was 20 months and 13 months for those treated with VIDAZA®, and these outcomes were negatively impacted by the presence of TP53 and ZRSR2 mutations. More patients in the DACOGEN® group experienced myelosuppression, and grade 3 toxicities were rare.
The authors concluded that lower doses of DACOGEN® and VIDAZA® are safe and effective in symptomatic patients with MDS, and DACOGEN® is more effective compared to VIDAZA®, in patients with higher-risk features. A randomized phase II study of low-dose decitabine versus low-dose azacitidine in lower risk MDS and MDS/MPN. Jabbour E, Short NJ, Montalban-Bravo G, et al. Blood. 2017 Aug 3. pii: blood-2017-06-788497. doi: 10.1182/blood-2017-06-788497. [Epub ahead of print]

