SUMMARY: ColoRectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimates that approximately 135,000 new cases of ColoRectal Cancer will be diagnosed in the United States in 2016 and over 49,000 patients are expected to die of the disease. Even though colon cancer localized to the bowel is potentially curable with surgery and adjuvant chemotherapy, advanced colon cancer is often incurable. Standard chemotherapy when combined with anti EGFR (Epidermal Growth Factor Receptor) targeted monoclonal antibodies such as VECTIBIX® (Panitumumab) and ERBITUX® (Cetuximab) as well as anti VEGF agent AVASTIN® (Bevacizumab), have demonstrated improvement in Progression Free Survival (PFS) and Overall Survival (OS). The benefit with anti EGFR agents however is only demonstrable in patients with metastatic CRC, whose tumors do not harbor KRAS mutations in codons 12 and 13 of exon 2 (KRAS Wild Type). It is now becoming clear that even among the KRAS Wild Type patients, about 15% to 20% have other rare mutations such as NRAS and BRAF mutations, which confer resistance to anti EGFR agents. Therefore, pan RAS (expanded RAS) testing is now recommended.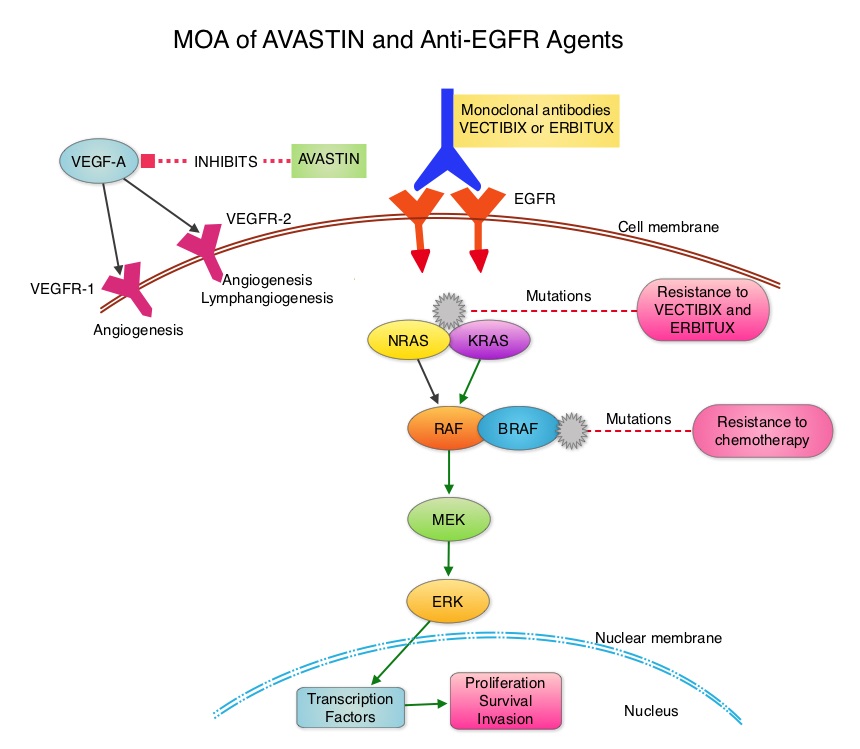
The CALGB/SWOG 80405 study reported that either ERBITUX® or AVASTIN® in combination with chemotherapy have equivalent Overall Survival benefit, when given as first line therapy, for patients with metastatic ColoRectal Cancer (mCRC), whose tumors are KRAS Wild Type. Consequently, the optimal first-line therapy for patients with KRAS Wild Type metastatic CRC still remains unclear. ERBITUX® has a similar activity across all treatment lines whereas AVASTIN® appears to lose its efficacy along the course of treatment lines. Although FOLFOX and FOLFIRI are equally effective as first line treatment for patients with metastatic CRC, FOLFOX might be more effective as second line treatment. Preclinical studies have suggested that a prior anti VEGF therapy, may lower sensitivity to a subsequent anti EGFR treatment.
The authors in a phase III randomized multicenter trial addressed these issues by comparing two different sequences of ERBITUX® and FOLFOX chemotherapy in KRAS Wild Type metastatic CRC patients, refractory to first line FOLFIRI chemotherapy and AVASTIN®. Patients with mCRC (N=110) were randomly assigned in a 1:1 ratio to receive CAMPTOSAR® (Irinotecan)/ERBITUX® as second line followed by FOLFOX-4 chemotherapy as third line (Arm A) or the reverse sequence (FOLFOX-4 chemotherapy second line followed by CAMPTOSAR®/ERBITUX® as third line – Arm B). The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS) and toxicity. It was noted that the median PFS in Arm A was 9.9 months compared to 11.3 months in Arm B (HR=0.85; P=0.42) and the median OS was 12.3 months in Arm A and 18.6 months in Arm B (HR=0.79; P=0.28). The Objective Response Rate in Arm A was 37% and in Arm B was 57% (P=0.05). Treatment was well tolerated with a low incidence of serious adverse events.
It was concluded that EGFR inhibition is not active immediately after VEGF blockade and therefore ERBITUX® is less effective immediately after AVASTIN®. The sequence of biological agents appears to be more important than the first-line chemotherapy choice. In RAS Wild Type metastatic CRC patients progressing after a first line AVASTIN® based therapy, ERBITUX® should be given in the third line setting or should be considered in first line treatment regimen. Efficacy of cetuximab immediately after bevacizumab: A phase III multicenter trial comparing two different sequences of cetuximab and FOLFOX in K-Ras WT metastatic colorectal cancer patients refractory FOLFIRI/bevacizumab. Cascinu S, Zaniboni A, Lonardi S, et al. J Clin Oncol 34, 2016 (suppl 4S; abstr 632)

