SUMMARY: The FDA on March 23, 2017, granted accelerated approval to BAVENCIO® (Avelumab) for the treatment of patients 12 years and older with metastatic Merkel Cell Carcinoma (MCC). Even though skin cancer is by far the most common type of cancer in the US, Merkel Cell Carcinoma (MCC) is not common. The American Cancer Society estimates that about 1,500 cases of MCC are diagnosed in the United States each year and over 90% of those diagnosed with MCC are older than 50yrs of age. The incidence of MCC in the US has tripled between 1986 and 2001. Merkel Cell Carcinoma, also known as Trabecular Cancer of the Skin, is much more common in Caucasians than in people of other races, and occurs at increased frequency in individuals who are immunodeficient including patients who are transplant recipients. Other risk factors include exposure to ultraviolet light.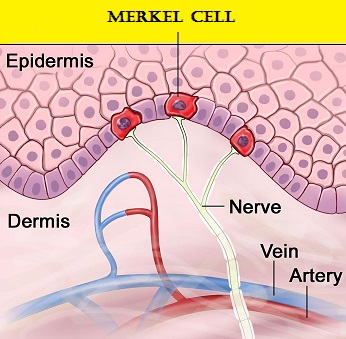
Merkel cells are often found at the base of the epidermis and are responsible for fine touch and pressure sensation. Merkel Cell Carcinoma (MCC) is a very aggressive neuroendocrine carcinoma of the skin. Merkel Cell PolyomaVirus (MCPyV) has been identified in over 80% of Merkel Cell tumors and a majority of MCCs contain clonally integrated viral DNA and express viral T antigen transcripts and protein. The presence of the virus in tumor cells can be confirmed by FISH analysis. This along with ultraviolet radiation-induced mutations provides the rationale for the treatment of MCC, with antibodies that target the PD-L1/PD-1 pathway. BAVENCIO® is a human IgG1 lambda monoclonal antibody, directed against Programmed cell Death Ligand1 (PD-L1) and blocks the interaction of PD-L1 with PD-1. By inhibiting checkpoint proteins and their ligands, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response. BAVENCIO® is the first FDA-approved product to treat Merkel Cell Carcinoma.
The approval of BAVENCIO® was based on data from the JAVELIN study which is a multicentre, international, prospective, open-label, phase II trial. This study enrolled 88 previously treated patients with metastatic Merkel Cell Carcinoma. The median age was 72 years. Visceral disease was present in over 50% of patients. Overall, 66% of patients were PD-L1-positive and 52% were positive for the Merkel Cell PolyomaVirus (MCPyV). BAVENCIO® was given intravenously at 10 mg/kg IV every 2 weeks. The Primary endpoint was Objective Response Rate (Complete Response or Partial Response), assessed by an Independent Review Committee. Safety and clinical activity were assessed in all patients who received at least one dose of study drug.
The Objective Response Rate was 33% with 11% Complete Response and 22% Partial Response. Among the responding patients, the response duration ranged from 3 months to 23 months with 86% of responses durable for 6 months or longer. Responses were ongoing in 82% of the patients at the time of analysis. Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel Cell PolyomaVirus. The most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema.
The authors concluded that BAVENCIO® is associated with durable response rates and represents a new therapeutic option for advanced Merkel Cell Carcinoma. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Kaufman H, Russell JS, Hamid O, et al. Lancet Oncol. 2016;17:1374-1385

