SUMMARY: The FDA on December 19, 2017 granted accelerated approval to BOSULIF® (Bosutinib) for treatment of patients with newly diagnosed Chronic Phase Philadelphia chromosome positive (Ph+) Chronic Myelogenous Leukemia (CML). Chronic Myeloid Leukemia (CML) constitutes a little over 10% of all new cases of leukemia. The American Cancer Society estimates that about 8,950 new CML cases will be diagnosed in the United States in 2017 and about 1,080 patients will die of the disease. The hallmark of CML, the Philadelphia Chromosome (Chromosome 22), is a result of a reciprocal translocation between chromosomes 9 and 22, wherein the ABL gene from chromosome 9 fuses with the BCR gene on chromosome 22. As a result, the auto inhibitory function of the ABL gene is lost and the BCR-ABL fusion gene is activated resulting in cell proliferation and leukemic transformation of hematopoietic stem cells. The presently available Tyrosine Kinase Inhibitors (TKI’s) approved in the United States including GLEEVEC® (Imatinib), share the same therapeutic target, which is BCR-ABL kinase. BOSULIF® is a potent, dual Abl and Src tyrosine kinase inhibitor and was approved by the FDA in 2012 for the treatment of adult patients with chronic, accelerated, or blast phase Philadelphia chromosome positive (Ph+) Chronic Myelogenous Leukemia (CML) with resistance or intolerance to prior therapy.
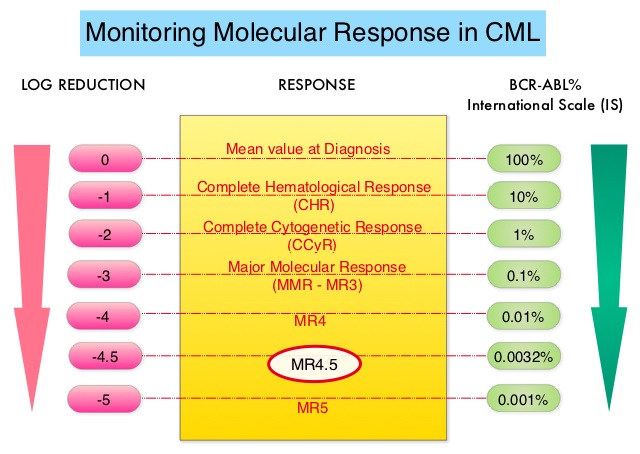 This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241). The median age of patients was 53 years, the Sokal risk group was intermediate and high for 40% and 21% of patients, respectively, and over two thirds of the patients had an ECOG PS score of 0. Sokal score is calculated using a formula that includes Age, Spleen size, Platelet count and percentage of Myeloblasts and has three risk groups: Low-risk (Sokal score<0.8), Intermediate-risk (Sokal score 0.8-1.2) and High-risk (Sokal score >1.2). The Primary endpoint was Major Molecular Response (MMR) at 12 months, defined as BCR-ABL ratio on International Scale of 0.1% or less, which corresponded to 3 or more log reduction from standardized baseline.
This new approval for BOSULIF® was based on the efficacy and safety data from BFORE trial, which is an ongoing randomized, open-label, multicenter, phase III study in which in 487 patients with Ph+ newly diagnosed Chronic Phase CML were randomized to receive either BOSULIF® 400 mg once daily (N=246) or GLEEVEC® (Imatinib) 400 mg once daily (N=241). The median age of patients was 53 years, the Sokal risk group was intermediate and high for 40% and 21% of patients, respectively, and over two thirds of the patients had an ECOG PS score of 0. Sokal score is calculated using a formula that includes Age, Spleen size, Platelet count and percentage of Myeloblasts and has three risk groups: Low-risk (Sokal score<0.8), Intermediate-risk (Sokal score 0.8-1.2) and High-risk (Sokal score >1.2). The Primary endpoint was Major Molecular Response (MMR) at 12 months, defined as BCR-ABL ratio on International Scale of 0.1% or less, which corresponded to 3 or more log reduction from standardized baseline.
After 12 or more months of follow up, the MMR at 12 months was 47.2% in the BOSULIF® group and 36.9% in the GLEEVEC® group (P=0.02) and the time to achieve a MMR was shorter in the BOSULIF® group (P<0.02). The Complete Cytogenetic Response (CCyR) at 12 months was also significantly higher with BOSULIF® versus GLEEVEC® (77.2% vs 66.4%; P< 0.008), with the time to achieve a CCyR, shorter for BOSULIF® (P<0.001). The most common adverse reactions in the BOSULIF® group included rash, nausea, diarrhea, abdominal discomfort, thrombocytopenia, increased ALT and AST levels.
It was concluded that BOSULIF® is an important and useful treatment option for patient with newly diagnosed Chronic Phase CML. Bosutinib (BOS) versus imatinib (IM) for newly diagnosed chronic myeloid leukemia (CML): Initial results from the BFORE trial. Cortes JE, Gambacorti-Passerini C, Deininger MWN, et al. J Clin Oncol. 2017;35 (suppl; abstr 7002).

