SUMMARY: The FDA on May 15, 2019 approved VENCLEXTA® (Venetoclax) for adult patients with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). The FDA in 2018 had approved VENCLEXTA® for patients with CLL or SLL with or without 17p deletion, who have received at least one prior therapy. The American Cancer Society estimates that for 2019, about 20,720 new cases of CLL will be diagnosed in the US and 3,930 patients will die of the disease. B-cell CLL is the most common type of leukemia in adults, accounting for about 11% of all hematologic malignancies.
The pro-survival (anti-apoptotic) protein BCL2 is over expressed by CLL cells and regulates clonal selection and cell survival. A new class of anticancer agents known as BH3-mimetic drugs mimic the activity of the physiologic antagonists of BCL2 and related proteins and promote apoptosis (programmed cell death). VENCLEXTA® is a second generation, oral, selective, small molecule inhibitor of BCL2 and restores the apoptotic processes in tumor cells. 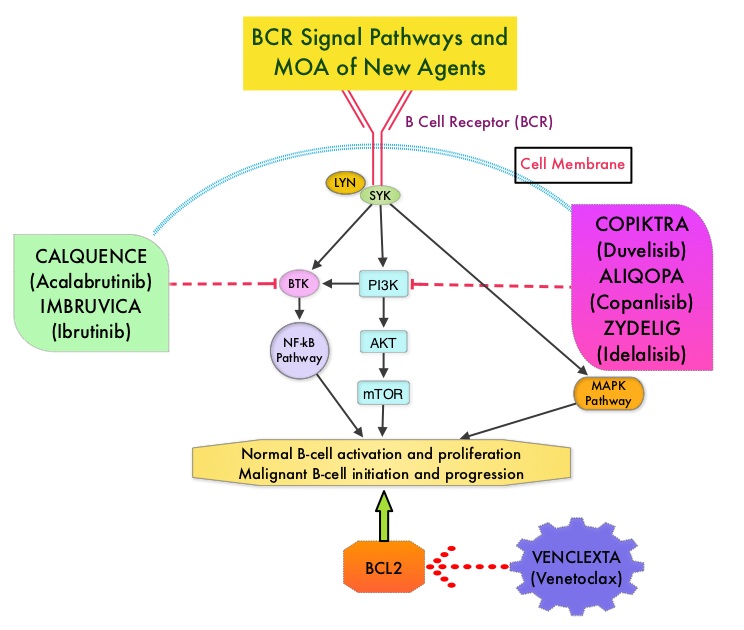
CLL14 Trial is a prospective, multicenter, open-label, randomized Phase III study, conducted in close collaboration with the German CLL Study Group (DCLLSG). This study was designed to evaluate the efficacy and safety of a fixed duration combination of VENCLEXTA® and GAZYVA® (Obinutuzumab) versus GAZYVA® and Chlorambucil in previously-untreated patients with CLL and coexisting medical conditions. In this trial, 432 treatment-naïve patients with CLL were randomized in a 1:1 ratio to receive fixed duration of 12 months of VENCLEXTA® in combination with six cycles of GAZYVA®, or 6 cycles of GAZYVA® in combination with Chlorambucil. Both treatment groups were well balanced and the median patient age was 72 years. The Primary endpoint was Progression Free Survival (PFS) assessed by an Independent Review Committee. Secondary endpoints included Minimal Residual Disease (MRD) status, Overall Response Rate, Complete Response, Complete Remission with Incomplete Hematologic Recovery (CRi), Overall Survival, duration of response, time to next CLL treatment, and safety.
The trial demonstrated a statistically significant improvement in PFS for patients who received VENCLEXTA® plus GAZYVA® compared with those who received GAZYVA® plus Chlorambucil (HR 0.33; P<0.0001), suggesting a 67% reduction in the risk of progression or death with the VENCLEXTA® plus GAZYVA® combination. The median PFS was not reached in either treatment groups after a median follow-up of 28 months. The Overall Response Rate was 85% in VENCLEXTA® plus GAZYVA® group compared to 71% in GAZYVA® plus Chlorambucil group (P=0.0007). The trial also demonstrated statistically significant improvements in rates of Minimal Residual Disease (MRD) negativity (less than one CLL cell per 104 leukocytes) in bone marrow and peripheral blood. The rate of MRD-negativity in the bone marrow was 57% in the VENCLEXTA® group compared with 17% in the GAZYVA® plus Chlorambucil group. The MRD-negativity rates in the peripheral blood were 76% versus 35%, respectively. Overall Survival data were not mature at this analysis. The most common adverse events in the VENCLEXTA® plus GAZYVA® group included neutropenia, thrombocytopenia, anemia, diarrhea, nausea, upper respiratory tract infection, cough, musculoskeletal pain, fatigue, and edema.
It was concluded that a combination of VENCLEXTA® and GAZYVA® among patients with previously untreated CLL significantly improved Progression Free Survival, compared to patients treated with standard of care GAZYVA® plus Chlorambucil. The authors added that VENCLEXTA® plus GAZYVA® is the only chemotherapy-free regimen of fixed duration, and is a major step forward in the management of previously untreated CLL patients. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-and-sll

