SUMMARY: The FDA on March 13, 2017 approved KISQALI® (Ribociclib), a cyclin-dependent kinase 4/6 inhibitor, in combination with an Aromatase Inhibitor, as initial endocrine-based therapy for the treatment of postmenopausal women with Hormone Receptor (HR)-positive, Human Epidermal growth factor Receptor 2 (HER2)-negative advanced or metastatic breast cancer. Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop breast cancer during their life time. Approximately, 255,180 new cases of breast cancer will be diagnosed in 2017 and 41,070 women will die of the disease. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. However, resistance to hormonal therapy occurs in a majority of the patients.
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6), phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors.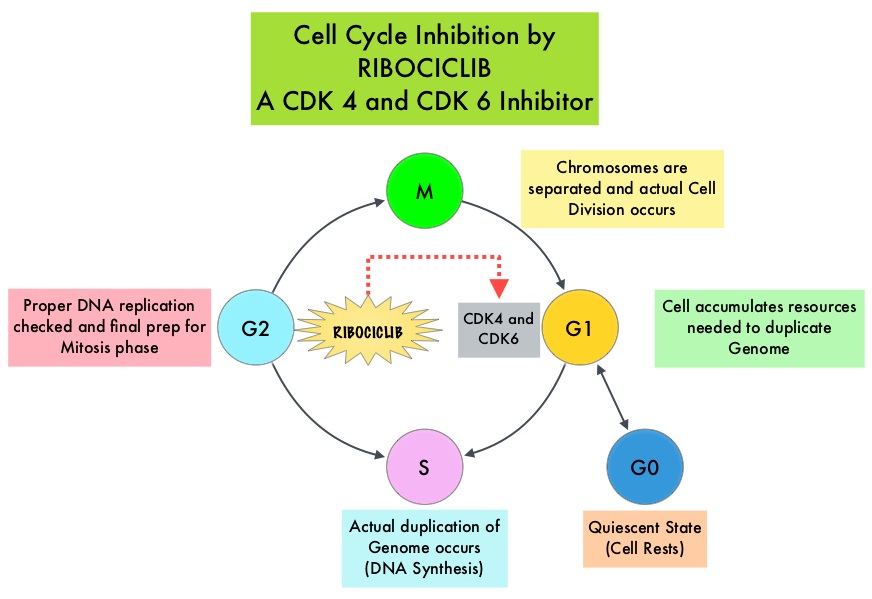
KISQALI® is an orally bioavailable, selective, small-molecule inhibitor of CDK4/6 that blocks the phosphorylation of RetinoBlastoma protein, thereby preventing cell-cycle progression and inducing G1 phase arrest. In a phase 1b study involving postmenopausal women with ER positive, HER2-negative advanced breast cancer, KISQALI® in combination with FEMARA® (Letrozole) demonstrated an Overall Response Rate (ORR) of 46% and a Clinical Benefit Rate of 79%, in treatment-naïve patients with advanced breast cancer.
MONALEESA-2 trial is a randomized, double-blind, placebo-controlled, phase III study in which 668 patients were randomly assigned in a 1:1 ratio to receive either KISQALI® plus FEMARA® (Letrozole) or placebo plus FEMARA®. Eligible patients included post-menopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer who had received no prior therapy for advanced disease. Treatment consisted of oral KISQALI® 600 mg daily on a 3-weeks on and 1-week off schedule, in 28-day treatment cycles plus FEMARA® 2.5 mg orally daily on a continuous schedule or placebo plus FEMARA®. Patients were stratified according to the presence or absence of liver or lung metastases and treatment was continued until disease progression or unacceptable toxicity. No treatment crossover was allowed. The median age was 62 years and close to 60% of the patients had visceral metastases. The primary end point was Progression Free Survival (PFS) and secondary end points included Overall Survival (OS), Overall Response Rate (ORR), Clinical Benefit Rate (Overall Response plus stable disease lasting 24 weeks or more), safety, and Quality of Life assessments.
A pre-planned interim efficacy analysis demonstrated a significant improvement in the PFS amongst the KISQALI® group compared to the placebo group (HR=0.56; P<0.0001). The median duration of follow-up was 15.3 months. The estimated median PFS had not been reached in the KISQALI® group and was 14.7 months in the placebo containing arm. The Overall Response Rate (ORR) in patients with measurable disease was 52.7% in the KISQALI® group and 37.1% in the placebo plus FEMARA® group (P<0.001). Overall Survival data was mature at the time of this analysis. The rates of discontinuation because of adverse events were 7.5% in the KISQALI® group and 2.1% in the placebo group. The most common adverse reactions observed in patients taking KISQALI® were myelosuppression, nausea, vomiting, diarrhea and fatigue, as well as abnormal liver function tests. KISQALI® has been shown to prolong the QT interval in a dose-dependent manner and prolongation of the QT interval occurred in 3.3% of patients treated at the 600 mg dose, with changes mostly occurring within the first 4 weeks of treatment.
The authors concluded that among patients receiving initial systemic treatment for HR-positive, HER2-negative advanced breast cancer, the addition of KISQALI® to FEMARA® significantly prolonged PFS compared to FEMARA® alone, with a higher rate of myelosuppression noted in the KISQALI® group. Ribociclib as First-Line Therapy for HR-positive, Advanced Breast Cancer. Hortobagyi GN, Stemmer SM, Burris HA, et al. N Engl J Med 375:1738-1748, 2016.

