SUMMARY: The FDA on October 18, 2016, approved TECENTRIQ® (Atezolizumab) for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose disease progressed during or following platinum-containing chemotherapy. Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2016 about 224,390 new cases of lung cancer will be diagnosed and over 158,000 patients will die of the disease. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. The treatment paradigm for malignancies has been rapidly evolving, with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, Immune checkpoints or gate keepers inhibit an intense immune response by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are now available that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), as well as Programmed cell Death Ligands (PD-L1), that are expressed by cells in the tumor micro environment. By targeting the Immune check point proteins or their ligands, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.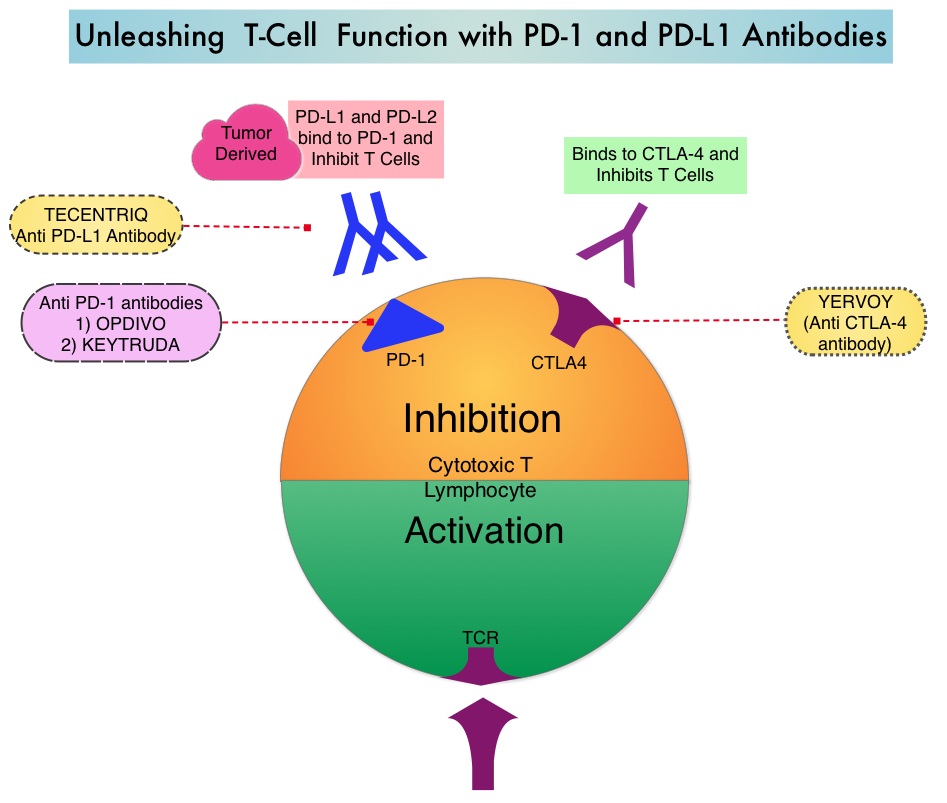
TECENTRIQ® (Atezolizumab) is an anti PD-L1 monoclonal antibody designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating Immune Cells, thereby blocking its interactions with PD-1 and B7.1 receptors and thus enabling the activation of T cells and restoring tumor-specific T-cell immunity. The approval of TECENTRIQ® was based two international, clinical trials (OAK and POPLAR trials). TECENTRIQ® demonstrated survival benefit compared to Docetaxel in a multicenter, randomized, phase II study (POPLAR trial). In this study, the median Overall Survival was 12.6 months and 9.7 months (HR=0.69) for the TECENTRIQ® and Docetaxel groups respectively.
OAK trial is a global, multicentre, open-label, randomized, controlled Phase III study in which 1225 patients with locally advanced or metastatic NSCLC, whose disease had progressed following previous treatment with platinum-containing chemotherapy, were enrolled. Patients with both squamous and non-squamous histology were randomized in a 1:1 ratio to receive either TECENTRIQ® 1200 mg IV every 3 weeks or Docetaxel 75 mg/m2 IV every 3 weeks. Patients were stratified according to PD-L1 status, number of prior chemotherapy regimens and histology. The median age was 64 years, 25% had 2 prior lines of therapy and 26% had squamous histology. The co-primary endpoints were Overall Survival (OS) in all randomized patients and in a PD-L1 selected subgroup in the primary analysis population. Secondary endpoints included Progression Free Survival, Objective Response Rate, Duration of Response and Safety.
The primary efficacy analysis was conducted and reported in the first 850 of 1225 total enrolled patients. The median Overall Survival was 13.8 months in the TECENTRIQ® group compared to 9.6 months in the Docetaxel group (HR=0.74; P=0.0004), with a 26% improvement in Overall Survival in the patient group who received TECENTRIQ®. This benefit was seen regardless of their PD-L1 expression levels, including patients whose tumors displayed PD-L1 expression of less than 1%. Patients with high PD-L1 expression had more pronounced benefit with TECENTRIQ® with a 59% improvement in OS compared with Docetaxel (HR=0.41; P<0.0001). The OS benefit was similar in patients with squamous or non-squamous histology. The most common adverse reactions in patients in patients treated with TECENTRIQ® were fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation.
The authors concluded that TECENTRIQ® offers a new second-line therapeutic strategy for patients with Non Small Cell Lung Cancer, with Overall Survival benefit, regardless of the PD-L1 status of the tumor. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Barlesi F, Park K, Ciardiello F et al. Abstract LBA44_PR. Presented at: 2016 ESMO Congress; October 7–11 (2016) Copenhagen, Denmark.

