SUMMARY: The American Cancer Society estimates that about 71,850 people will be diagnosed with Non-Hodgkin Lymphoma (NHL) in the United States and about 19,800 individuals will die of this disease. Approximately 20% of all NHLs are Follicular Lymphomas (FL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Advanced stage Follicular lymphomas are not curable and as such prolonging Progression Free Survival (PFS) and Overall Survival (OS) while maintaining quality of life (QoL), has been the goals of treatment intervention. Asymptomatic patients with FL are generally considered candidates for “watch and wait” approach, whereas those with B symptoms (fever, night sweats, and weight loss), painful lymphadenopathy/splenomegaly, organ compromise and cytopenias are generally considered candidates for therapy. Follicular Lymphoma International Prognostic Index (FLIPI) is of prognostic value and is used to help with treatment choices. The Ann Arbor classification divides FL into four stages. Patients with stages I and II have localized disease and those with stages III and IV have advanced disease. The World Health Organization (WHO) further classified FL based on histology into low grade (grades 1 and 2) and high grade (grade 3a) FLs. Grade 3b FL which demonstrates diffuse areas of involvement is designated as Diffuse Large B-cell Lymphoma (DLBCL) and is treated as such.
The authors in this publication following review of literature from 2000-2014, addressed FOUR important questions
What treatment options should be considered for localized FL?
Recommendation 1: The preferred treatment for localized FL is Radiation Therapy (RT).
Recommendation 2: Given the potential for long term toxicities, lower doses of RT (24 to 30 Gy) in 1.5 to 2Gy fractions and smaller field sizes, is recommended.
Recommendation 3: Combined modality treatments may be considered for patients with localized disease if positive outcomes are proven in randomized studies.
Recommendation 4: Observation alone may be a reasonable alternative if the potential toxicity of RT outweighs the potential benefits or if the patient refuses RT.
How should asymptomatic advanced-stage FL be managed?
Recommendation 1: Initiation of Chemotherapy or Immunotherapy should be based on the identification of symptoms as defined by the GELF or BNLI criteria.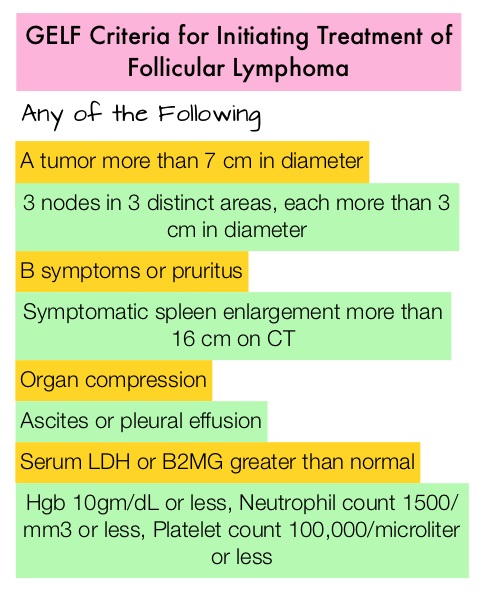
Recommendation 2: Chemoimmunotherapy or Chemotherapy alone as early treatment is not recommended in asymptomatic advanced FL, because of the lack of published randomized studies. Observation alone is therefore recommended in patients who do not meet the criteria for treatment with Chemotherapy or Immunotherapy.
Recommendation 3: If ongoing randomized studies show positive outcomes with reduced risk of relapse, early treatment with RITUXAN® (Rituximab) with or without RITUXAN® maintenance in asymptomatic patients may be considered.
What treatment options should be considered for symptomatic advanced-stage FL?
Recommendation 1: Based on the lower response rate with RITUXAN® monotherapy, Chemoimmunotherapy is preferred over RITUXAN® monotherapy , for the first-line treatment of symptomatic advanced stage FL, except when Chemotherapy is contraindicated.
Recommendation 2: RITUXAN® should be added to chemotherapy in the first line treatment of symptomatic advanced-stage FL given the improved Overall Response Rate (ORR) and Progression Free survival (PFS) demonstrated with the addition of RITUXAN® to a number of Chemotherapy combinations.
Recommendation 3: TREANDA® (Bendamustine) and RITUXAN® (BR) is the preferred Chemoimmunotherapy regimen for the first-line treatment of symptomatic advanced-stage FL given the superior efficacy and favorable tolerability of this regimen compared to R-CHOP, confirmed in 2 randomized trials.
In which patients should additional treatment be considered (ie, maintenance, consolidation, SCT)?
Recommendation 1: Maintenance RITUXAN® is recommended after first-line treatment of FL given the improved response with RITUXAN® maintenance versus observation, demonstrated in 2 randomized trials. The optimal frequency and duration of maintenance RITUXAN® is presently unclear.
Recommendation 2: High Dose Therapy (HDT) followed by Autologous Stem Cell Transplantation (ASCT) is not recommended as part of front-line treatment of FL, given the lack of a survival benefit and the potential toxicity of this approach. There also is no evidence to support the use of Radioimmunotherapy (RIT) after first-line treatment of FL.
A Canadian Evidence-Based Guideline for the First-Line Treatment of Follicular Lymphoma: Joint Consensus of the Lymphoma Canada Scientific Advisory Board. Kuruvilla J, Assouline S, Hodgson D, et al. Clinical Lymphoma Myeloma and Leukemia 2015; 15:59-74

