SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. About 268,600 new cases of female breast cancer will be diagnosed in 2019 and about 41,760 women will die of the disease. Breast cancer is the second leading cause of cancer death in the US. Approximately 70% of breast tumors express Estrogen Receptors and/or Progesterone Receptors and these patients are often treated with anti-estrogen therapy as first line treatment. The incidence of breast cancer among women under the age of 50 has been increasing by 0.2% per year. Premenopausal breast cancer may be biologically different than post menopausal breast cancer and diagnosis of breast cancer at a young age has been associated with adverse outcomes and less sensitivity to endocrine therapy. Further, premenopausal women are often excluded from hormone therapy trials. The incidence of metastatic disease at the time of diagnosis among patients with Hormone Receptor (HR)- positive breast cancer, has been increasing by about 2% per year.
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in HR-positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors.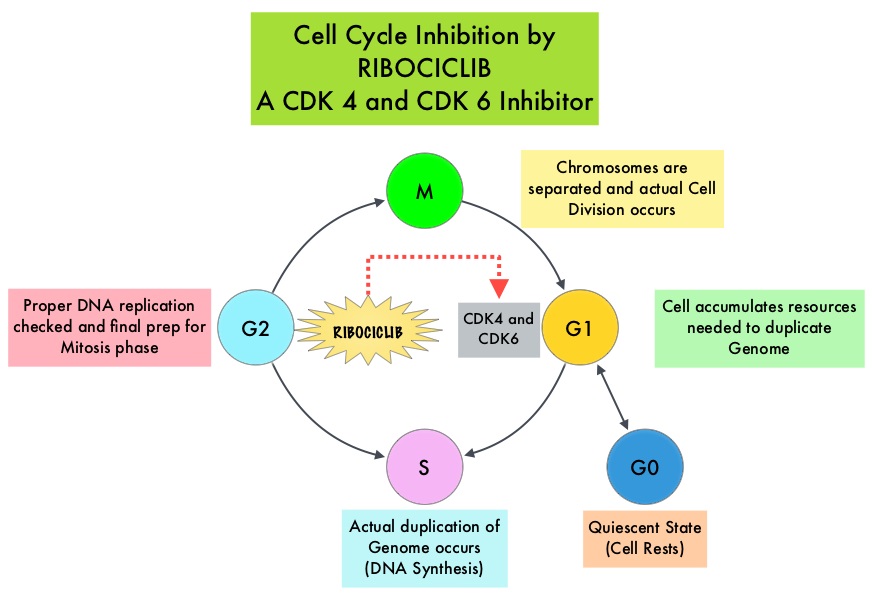
There are presently three CDK4/6 inhibitors approved by the FDA and they include KISQALI® (Ribociclib), IBRANCE® (Palbociclib) and VERZENIO® (Abemaciclib). All three agents have demonstrated similar, significantly prolonged Progression Free Survival (PFS) when administered in combination with endocrine therapy, as first-line treatment, in women with HR-positive metastatic breast cancer (MONALEESA-2 with KISQALI®, PALOMA-2 with IBRANCE® and MONARCH-3 with VERZENIO®). These trials for the first-line treatment of advanced breast cancer however excluded premenopausal women. The toxicities were slightly different with neutropenia more commonly encountered in the IBRANCE® and KISQALI® studies and diarrhea more often noted with VERZENIO®. KISQALI® (Ribociclib) is an orally bioavailable, selective, small-molecule inhibitor of CDK4/6 that blocks the phosphorylation of RetinoBlastoma protein, thereby preventing cell-cycle progression and inducing G1 phase arrest. It is four times more selective for CDK4 than for CDK6.
The MONALEESA-7 trial is an international, randomized, double-blind, placebo-controlled, Phase III trial in which KISQALI® in combination with endocrine therapy was compared with placebo in combination with endocrine therapy, in premenopausal or perimenopausal women with HR-positive, HER2- negative advanced breast cancer. Patients (N=672) were randomly assigned in a 1:1 ratio, to receive KISQALI® at 600 mg orally once daily for 21 days of each 28 day cycle (N=335), or matching placebo (N=337). Both groups received ZOLADEX® (Goserelin) 3.6 mg administered subcutaneously on day 1 of each 28 day cycle. Patients also received either a nonsteroidal Aromatase Inhibitor (Letrozole 2.5 mg or Anastrozole 1 mg) or Tamoxifen 20 mg, orally once daily continuously. The choice of endocrine therapy was made on the basis of the patient’s previous adjuvant or neoadjuvant therapy or investigator or patient preference. Crossover was not permitted between the two treatment groups. Patients were stratified according to the presence or absence of liver or lung metastases, previous chemotherapy for advanced disease and endocrine therapy. The Primary end point was Progression Free Survival (PFS) and Secondary endpoint included Overall Survival (OS). The superior PFS data with KISQALI® compared to endocrine therapy alone, was previously reported. The authors herein report the results on Overall Survival.
After a median follow up of 34.6 months, the addition of KISQALI® to endocrine therapy resulted in significantly longer Overall Survival, compared to endocrine therapy alone. The estimated OS at 42 months was 70.2% in the KISQALI® group and 46.0% in the placebo group (HR for death=0.71; P=0.00973), suggesting a 29% reduction in the risk of death. No new safety signals were observed and in the KISQALI® group, more instances of QT-interval prolongation were observed in patients who received Tamoxifen than in those who received an Aromatase Inhibitor, but without symptomatic arrhythmias or Torsades de pointes.
It was concluded that KISQALI® along with endocrine therapy significantly prolonged Overall Survival, compared to endocrine therapy alone, among pre and perimenopausal patients with advanced HR-positive, HER2-negative breast cancer and these findings represent a major treatment advance in this patient group. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. Im S-A, Lu Y-S, Bardia A, et al. N Engl J Med 2019; 381:307-316.

