SUMMARY: Aging Male Syndrome (AMS), also known as late-onset hypogonadism is a common condition associated with low testosterone levels and symptoms and signs of hypogonadism. They include weight gain, insomnia, irritability and mood swings, fatigue, loss of libido, loss of motivation, problems with memory and concentration, bone loss, loss of muscle mass and anemia. There is ample evidence suggesting that testosterone replacement therapy improves quality of life in men with Aging Male Syndrome. A definite correlation has not been established, between adjusted testosterone levels in hypogonadal men using testosterone replacement therapy and the initiation and/or promotion of latent prostate cancer.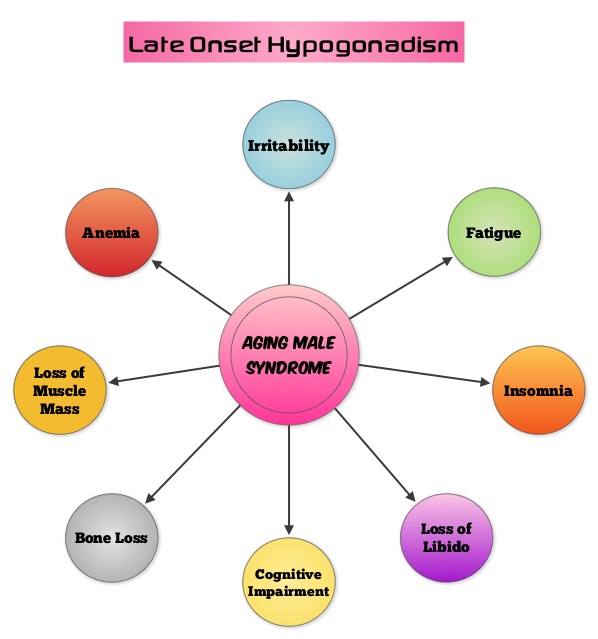 Based on the recent research, it appears that there is an indirect link between mutations of the Androgen Receptor gene and the initiation and promotion of prostate cancer. On the contrary, low testosterone levels prior to therapy may be an independent predictor of a more aggressive disease, with an increased likelihood of extra-prostatic disease at the time of diagnosis and unfavorable treatment response. The current recommendations are to exclude prostate cancer before initiating testosterone replacement therapy in hypogonadal men over age 40, with a digital rectal examination (DRE) and PSA level and to closely monitor in the first year of testosterone replacement with DRE and PSA evaluations every 3 months and then semiannually. These recommendations are arbitrary, and not supported by published data. To determine the incidence/risk of prostate cancer with testosterone replacement therapy, the authors in this study reviewed the outcomes of 942 men in three cohorts, with testosterone levels less than or equal to 12.1 nmol/L from three German centers, who had received testosterone undecanoate for up to 16 years. The incidence of prostate cancer in cohort A (N=300) was 39.4 per 10,000 person-years (39.4 cases per 10,000 persons followed for 1 year), in cohort B (N=261) was 54.5 per 10,000 person-years (54.5 cases per 10,000 persons followed for 1 year) and in cohort C (N=381), no person was diagnosed with prostate cancer. The authors pointed out that the incidence of prostate cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was 116 per 10,000 person-years. Even though this review of registry data cannot be directly compared with screening trials, the authors concluded that based on their registry studies, long-term testosterone treatment in hypogonadal men does not appear to increase prostate cancer risk. A recently published article, in the Annals of Pharmacotherapy by Baillargeon, et al. concluded that there was no increased risk of myocardial infarction when hypogonadal patients over age 65, were treated with intramuscular testosterone. As the debate continues on the risk/ benefits of testosterone replacement therapy, the heightened awareness will probably bring about more responsible prescribing of testosterone supplements. Haider A, Zitzmann M, Yassin A. J Clin Oncol 32, 2014 (suppl 4; abstr 119)
Based on the recent research, it appears that there is an indirect link between mutations of the Androgen Receptor gene and the initiation and promotion of prostate cancer. On the contrary, low testosterone levels prior to therapy may be an independent predictor of a more aggressive disease, with an increased likelihood of extra-prostatic disease at the time of diagnosis and unfavorable treatment response. The current recommendations are to exclude prostate cancer before initiating testosterone replacement therapy in hypogonadal men over age 40, with a digital rectal examination (DRE) and PSA level and to closely monitor in the first year of testosterone replacement with DRE and PSA evaluations every 3 months and then semiannually. These recommendations are arbitrary, and not supported by published data. To determine the incidence/risk of prostate cancer with testosterone replacement therapy, the authors in this study reviewed the outcomes of 942 men in three cohorts, with testosterone levels less than or equal to 12.1 nmol/L from three German centers, who had received testosterone undecanoate for up to 16 years. The incidence of prostate cancer in cohort A (N=300) was 39.4 per 10,000 person-years (39.4 cases per 10,000 persons followed for 1 year), in cohort B (N=261) was 54.5 per 10,000 person-years (54.5 cases per 10,000 persons followed for 1 year) and in cohort C (N=381), no person was diagnosed with prostate cancer. The authors pointed out that the incidence of prostate cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was 116 per 10,000 person-years. Even though this review of registry data cannot be directly compared with screening trials, the authors concluded that based on their registry studies, long-term testosterone treatment in hypogonadal men does not appear to increase prostate cancer risk. A recently published article, in the Annals of Pharmacotherapy by Baillargeon, et al. concluded that there was no increased risk of myocardial infarction when hypogonadal patients over age 65, were treated with intramuscular testosterone. As the debate continues on the risk/ benefits of testosterone replacement therapy, the heightened awareness will probably bring about more responsible prescribing of testosterone supplements. Haider A, Zitzmann M, Yassin A. J Clin Oncol 32, 2014 (suppl 4; abstr 119)

