SUMMARY: There is now growing body of evidence suggesting superior outcomes when advanced NSCLC patients with specific genomic alterations receive targeted therapies. Following review of 127 studies by experts and input from a scientific advisory panel, The College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP) offered evidence-based recommendations for the molecular analysis of lung cancers for Epidermal Growth Factor Receptor (EGFR ) mutations and Anaplastic Lymphoma Kinase (ALK) rearrangements, thereby selecting patients with lung cancer, for treatment with EGFR and ALK tyrosine kinase inhibitors. The ASCO review panel has endorsed these guidelines which specifically address the following questions:
1) Which patients should be tested for EGFR mutations and ALK rearrangements?
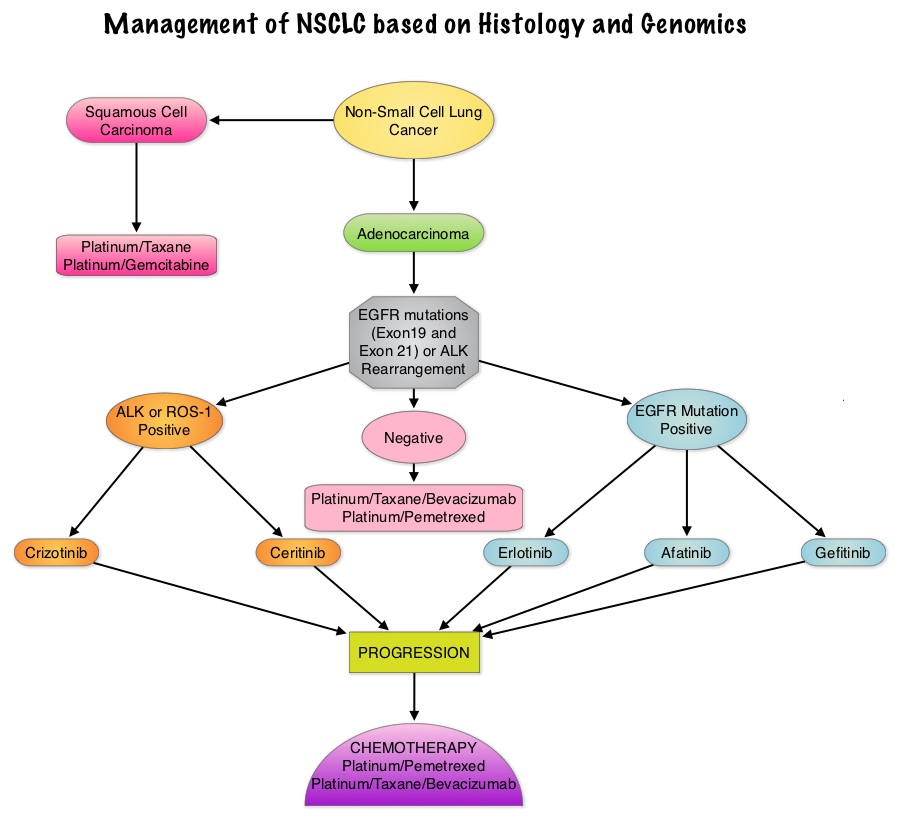
EGFR or ALK testing is recommended for all patients with advanced lung adenocarcinoma or tumors with an adenocarcinoma component, irrespective of clinical characteristics such as smoking history, sex, race, or other clinical factors. Tumor samples of other histologies for which an adenocarcinoma component cannot be excluded because of sampling, can be considered for testing, particularly if clinical criteria are suggestive (eg, younger age, lack of smoking history). Both primary tumors and metastatic lesions are suitable for testing. When fully excised lung cancer specimens are available, EGFR and ALK testing is not recommended in lung cancers that lack any adenocarcinoma component, such as pure squamous cell carcinomas, pure small-cell carcinomas, or large-cell carcinomas lacking IHC (ImmunoHistoChemistry) evidence of adenocarcinoma differentiation.
2) When should a patient specimen be tested for EGFR mutation or ALK rearrangement?
Testing should be ordered at the time of diagnosis of advanced disease or recurrence. For patients with earlier stage disease who undergo surgical resection, testing at the time of diagnosis is encouraged so that molecular information is available to an oncologist at the time of recurrence, for a subset of patients who subsequently experience recurrence. Tissue should be prioritized for EGFR and ALK testing.
 3) How rapidly should test results be available?
3) How rapidly should test results be available?
Laboratory turnaround times of 5 to 10 working days (2 weeks) for EGFR and ALK results are recommended.
4) How should specimens be processed for EGFR mutation testing?
Pathologists should use Formalin-Fixed, Paraffin-Embedded (FFPE) specimens or fresh frozen or alcohol-fixed specimens for PCR based EGFR mutation tests. EGFR and ALK testing can be performed with cytology samples, with cell blocks being preferred over smear preparations.
5) How should EGFR testing be performed?
EGFR testing should detect mutations in samples composed of as few as 50% tumor cells, although sensitivity to detect mutations in samples containing > 10% tumor cells is strongly encouraged. Sensitizing EGFR mutations with a population frequency of at least 1% should be reported. IHC for total EGFR as well as EGFR copy number analysis by FISH (Fluorescence In Situ Hybridization) is not recommended.
6) What is the role of KRAS analysis in selecting patients for targeted therapy with EGFR TKIs?
KRAS mutations are common (30%) in lung adenocarcinomas and mutually exclusive with EGFR and ALK. Testing for KRAS may be performed initially to exclude KRAS mutated tumors from EGFR and ALK testing but KRAS mutation testing is not recommended as a sole determinant of EGFR-targeted therapy.
7) What additional testing considerations are important in the setting of secondary or acquired EGFR TKI resistance?
If a laboratory performs testing on specimens from patients with acquired resistance to EGFR kinase inhibitors, such tests should be able to detect the secondary EGFR T790M mutation in as few as 5% of cells.
8) What methods should be used for ALK testing?
ALK FISH assay using dual labeled break-apart probes should be used for selecting patients for ALK TKI therapy. ALK IHC, if carefully validated, may be considered as a screening methodology to select specimens for ALK FISH testing. RT-PCR (Reverse Transcription–Polymerase Chain Reaction) is not recommended as an alternative to FISH, for selecting patients for ALK inhibitor therapy.
9) Are other molecular markers suitable for testing in lung cancer?
Testing for EGFR should be prioritized over other molecular markers in lung adenocarcinoma followed by testing for ALK. Testing for ROS1 and RET rearrangements may soon become a part of the guidelines.
10) How should molecular testing of lung adenocarcinomas be implemented and operationalized?
Pathology departments should establish a process wherein tissue (blocks or unstained slides) is sent to outside molecular laboratories within 3 days of receiving a request and to in house molecular laboratories within 24 hours. Results should be available within 2 weeks and reported in a format that is easily understood by oncologists and nonspecialist pathologists.
Leighl NB, Rekhtman N, Biermann WA, et al. J Clin Oncol 2014;32:3673-3679

