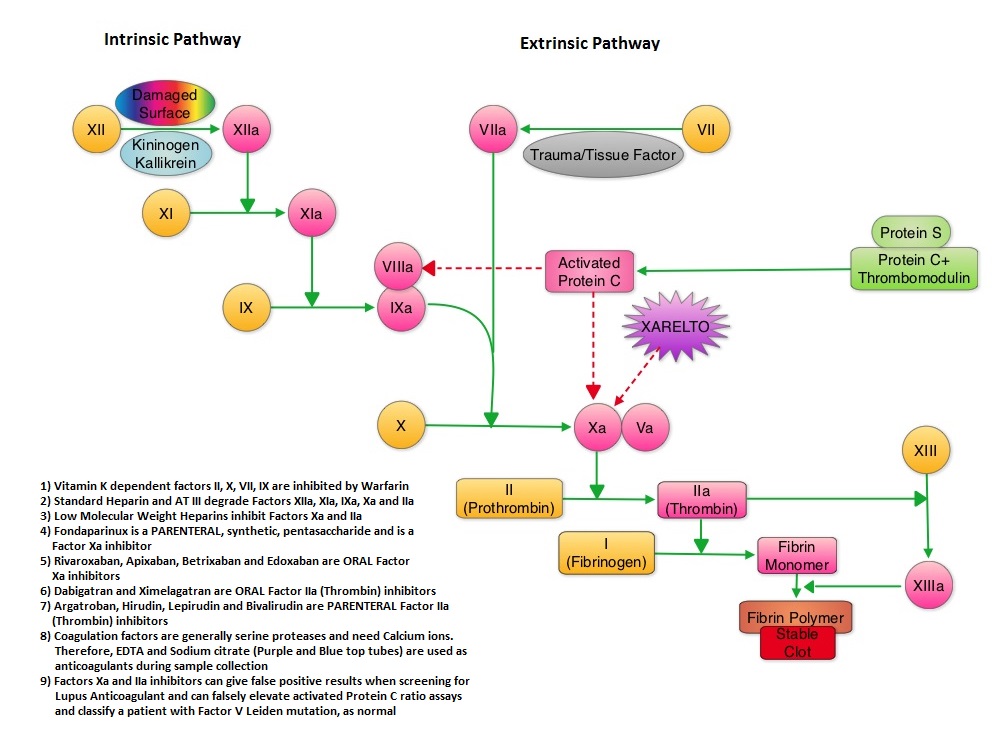
SUMMARY: XARELTO® (Rivaroxaban) is an oral, direct inhibitor of Factor Xa. Two previously published randomized studies concluded that XARELTO® was noninferior to the standard anticoagulation therapy (administration of heparin overlapped and followed by Vitamin K Antagonist), for most patients with Venous ThromboEmbolism. Further, XARELTO® in these studies also had a better safety profile compared to standard anticoagulant therapy. The authors in this pooled analysis combined the data from 2 identically designed studies, EINSTEIN-DVT and EINSTEIN-PE in which XARELTO® was compared to standard anticoagulation therapy, in patients with DVT and PE respectively. The goal of this study was to provide a more accurate estimate of the efficacy and safety of XARELTO® in elderly patients and cancer patients in whom Vitamin K Antagonists (VKA) such as COUMADIN® (Warfarin) can be associated with a higher complication rate. In addition, the study included analysis of outcomes with XARELTO® in patients with previous VTE and in those presenting with extensive thrombosis. Of the 8282 patients with VTE randomized in the pooled analysis, 4151 patients received XARELTO® and 4131 patients received LOVENOX® (Enoxaparin) / Vitamin K Antagonists. In both pooled studies, patients in the XARELTO® group received a dose of 15 mg PO BID for 21 days, followed by 20 mg PO QD whereas patients assigned to the standard anticoagulation therapy group received LOVENOX® subcutaneous at a dose of 1.0 mg/kg BID and either oral Warfarin or Acenocoumarol started within 48 hours after randomization and patients continued treatment for 3, 6, or 12 months, as determined by the local Health Care Providers. INR was closely monitored and maintained between 2-3. On final review, the analysis suggested that XARELTO® resulted in efficacy similar to standard anticoagulation therapy, with a noninferiority P<0.001. The pre-specified principal safety outcome was clinically relevant bleeding and major bleeding was observed in 40 patients belonging to the XARELTO® group and in 72 patients belonging to the standard anticoagulation therapy group (HR=0.54; P= 0.002). Similar benefits in the efficacy and safety were seen in the key subgroups evaluated, which included elderly fragile patients, cancer patients, patients presenting with extensive thrombosis and those with a history of recurrent VTE. The authors concluded that the incidence of major bleeding with XARELTO® was significantly less, particularly in the high risk group of patients, when compared to standard anticoagulation therapy and may therefore have a safety advantage, without compromising efficacy. Prins MH, Lensing AW, Bauersachs R, et al. Thrombosis Journal 2013;11:21