SUMMARY: Non-Hodgkin Lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. PI3K delta signaling is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. PI3K (PhosphatidylInositol 3-Kinase) is a lipid kinase and has four distinct isoforms – alpha, beta, gamma and delta. The alpha and beta isoforms are expressed in a wide variety of tissues whereas the gamma and delta isoforms are only expressed in hematopoietic cells. PI3K delta (the delta isoform of PI3K enzyme) is predominantly expressed in leukocytes and plays an important role in the normal B lymphocyte development as well as signal transduction from B cell receptor as well as receptors for various cytokines and chemokines.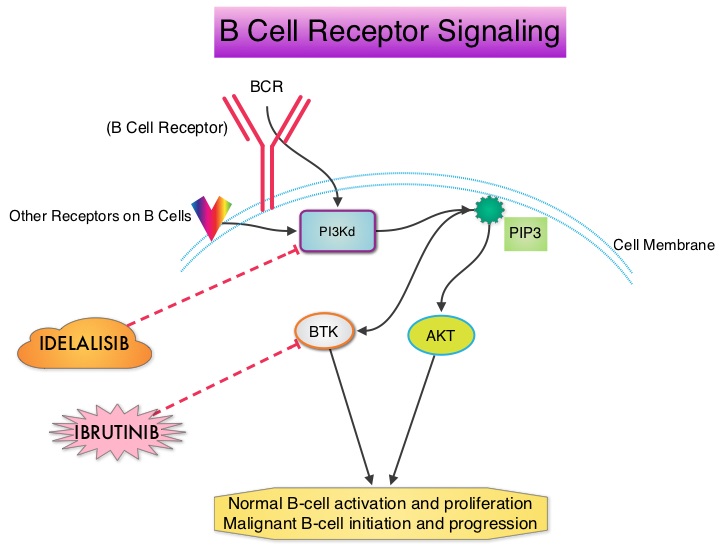 PI3K delta is activated by BCR signaling resulting in the production of a second messenger, Phosphatidylinositol 3,4,5-triphosphate (PIP3) which in turn activates Bruton’s Tyrosine Kinase (BTK) and AKT, a prosurvival kinase. Idelalisib (ZYDELIG®) is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. The FDA granted ZYDELIG® accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma based on the results of a single-arm, open-label, phase II trial. Patients with indolent Non-Hodgkin Lymphomas (N=125), who were refractory to Rituximab (RITUXAN®) and an alkylating agent or had relapsed within 6 months after receipt of these therapies, received ZYDELIG®, 150 mg PO BID. Treatment was continued until disease progression or unacceptable toxicities developed. The median age was 64 years and enrolled patients had received a median of four prior therapies. The indolent Non-Hodgkin Lymphoma subtypes included Follicular lymphoma (N=72), Small Lymphocytic Lymphoma (N=28), Marginal Zone Lymphoma (N=15), and Lymphoplasmacytic Lymphoma with or without Waldenström's Macroglobulinemia (N=10). The primary end point of this study was Overall Response Rate and secondary end points included the Duration of Response, Progression Free Survival, and Safety. The median follow up was 9.7 months. The Overall Response Rate was 57% with 50% partial responses and 6% complete responses. There was no difference in the Reponse Rates across the various subtypes of Indolent Non-Hodgkin Lymphomas. The median time to response was 1.9 months and the median duration of response was 12.5 months. The median Progression Free Survival was 11 months and the Overall Survival at one year was estimated to be 80%. The most common grade 3 or higher adverse events were diarrhea (13%), neutropenia (27%) and elevations in SGOT and SGPT levels (13%). These toxicities were manageable with dose modifications and dose interruptions. The authors concluded that ZYDELIG® has significant single agent activity, with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. Gopal AK, Kahl BS, de Vos S, et al. N Engl J Med 2014; 370:1008-1018
PI3K delta is activated by BCR signaling resulting in the production of a second messenger, Phosphatidylinositol 3,4,5-triphosphate (PIP3) which in turn activates Bruton’s Tyrosine Kinase (BTK) and AKT, a prosurvival kinase. Idelalisib (ZYDELIG®) is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. The FDA granted ZYDELIG® accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma based on the results of a single-arm, open-label, phase II trial. Patients with indolent Non-Hodgkin Lymphomas (N=125), who were refractory to Rituximab (RITUXAN®) and an alkylating agent or had relapsed within 6 months after receipt of these therapies, received ZYDELIG®, 150 mg PO BID. Treatment was continued until disease progression or unacceptable toxicities developed. The median age was 64 years and enrolled patients had received a median of four prior therapies. The indolent Non-Hodgkin Lymphoma subtypes included Follicular lymphoma (N=72), Small Lymphocytic Lymphoma (N=28), Marginal Zone Lymphoma (N=15), and Lymphoplasmacytic Lymphoma with or without Waldenström's Macroglobulinemia (N=10). The primary end point of this study was Overall Response Rate and secondary end points included the Duration of Response, Progression Free Survival, and Safety. The median follow up was 9.7 months. The Overall Response Rate was 57% with 50% partial responses and 6% complete responses. There was no difference in the Reponse Rates across the various subtypes of Indolent Non-Hodgkin Lymphomas. The median time to response was 1.9 months and the median duration of response was 12.5 months. The median Progression Free Survival was 11 months and the Overall Survival at one year was estimated to be 80%. The most common grade 3 or higher adverse events were diarrhea (13%), neutropenia (27%) and elevations in SGOT and SGPT levels (13%). These toxicities were manageable with dose modifications and dose interruptions. The authors concluded that ZYDELIG® has significant single agent activity, with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. Gopal AK, Kahl BS, de Vos S, et al. N Engl J Med 2014; 370:1008-1018

