SUMMARY: It is estimated that in the United States, approximately 22,530 women will be diagnosed with ovarian cancer in 2019 and 13,980 women will die of the disease. Ovarian cancer ranks fifth in cancer deaths among women, and accounts for more deaths than any other cancer of the female reproductive system. Approximately 75% of the ovarian cancer patients are diagnosed with advanced disease. Patients with newly diagnosed advanced ovarian cancer are often treated with platinum based chemotherapy following primary surgical cytoreduction. Approximately 70% of these patients will relapse within the subsequent 3 years and are incurable, with a 5 year Overall Survival rate of about 20-30%. 
BRCA1 and BRCA2 are tumor suppressor genes and functional BRCA proteins that repair damaged DNA, and play an important role in maintaining cellular genetic integrity. They regulate cell growth and prevent abnormal cell division and development of malignancy. Mutations in BRCA1 and BRCA2 account for about 20-25% of hereditary breast cancers and about 5-10% of all breast cancers. They also account for 15% of ovarian cancers, in addition to other cancers such as colon and prostate. BRCA mutations can either be inherited (Germline) and present in all individual cells or can be acquired and occur exclusively in the tumor cells (Somatic). Somatic mutations account for a significant portion of overall BRCA1 and BRCA2 aberrations. Loss of BRCA function due to frequent somatic aberrations in ovarian cancers likely deregulates Homologous Recombination (HR) pathway and increases sensitivity to platinum drugs. Majority of the women with Germline BRCA mutations (gBRCA) are positive for HR deficiency. The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. PARP inhibitors kill tumors defective in the BRCA1 or BRCA2 genes through the concept of synthetic lethality. Epithelial ovarian cancers with Homologous Recombination Deficiency have demonstrated sensitivity to PARP inhibitors.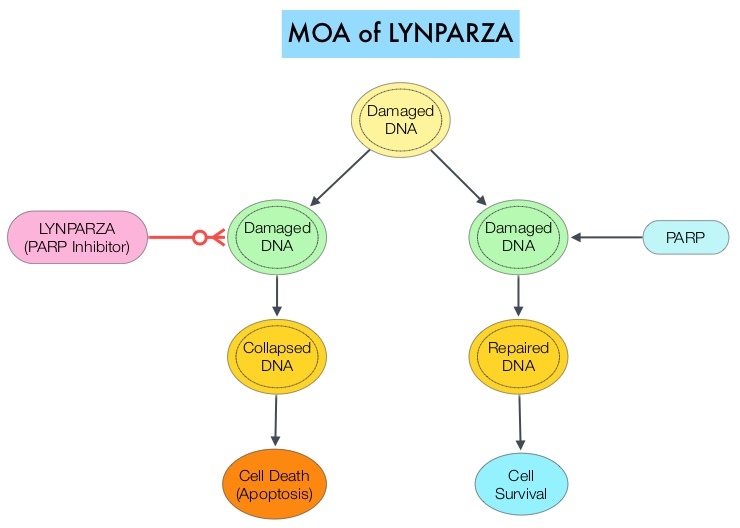
SOLO1 is an international, randomized, double-blind, Phase III trial, conducted to evaluate the efficacy of maintenance therapy with a PARP inhibitor LYNPARZA® (Olaparib), in patients with newly diagnosed advanced ovarian cancer with a Germline or Somatic mutation in BRCA1, BRCA2, or both (BRCA1/2), who had a complete or partial clinical response after platinum-based chemotherapy. Patients (N=391) were randomly assigned in a 2:1 ratio, to receive LYNPARZA® tablets 300 mg PO twice daily (N=260) or placebo (N=131). Enrolled patients had international FIGO Stage III or IV high-grade Serous or Endometrioid ovarian cancer, Primary Peritoneal cancer, or Fallopian tube cancer (or a combination thereof), and majority of the enrolled patients (N=388) had a centrally confirmed Germline BRCA1/2 mutation, and 2 patients had a centrally confirmed Somatic BRCA1/2 mutation. Patients with Stage III disease had cytoreductive surgery attempted upfront before the start chemotherapy, or had interval cytoreductive surgery after the start but before the end of chemotherapy. Patients with Stage IV disease either had a biopsy for tissue diagnosis or underwent upfront or interval cytoreductive surgery. The Primary end point was Progression Free Survival (PFS) and Secondary end points included second PFS which was the time from randomization to second disease progression or death, and Overall Survival.
After a median follow up of 41 months, the risk of disease progression or death was 70% lower with LYNPARZA® when compared to placebo, with an estimated rate of freedom from disease progression and death at 3 years of 60% versus 27% (HR for disease progression or death= 0.30; P<0.001). The estimated rate of freedom from second disease progression and death at 3 years was 75% in the LYNPARZA® group, as compared with 60% in the placebo group (HR for second disease progression or death=0.50; P<0.001). Adverse events were consistent with the known toxic effects of LYNPARZA®, with anemia being the most common serious side effect. Adverse events were usually managed by dose interruption or dose reduction, rather than discontinuation of the study drug.
It was concluded that maintenance therapy with LYNPARZA® after platinum-based chemotherapy provided a substantial Progression Free Survival benefit, with a 70% lower risk of disease progression or death, when compared to placebo, among women with newly diagnosed advanced ovarian cancer and a BRCA1/2mutation. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. Moore K, Colombo N, Scambia G, et al. N Engl J Med 2018; 379:2495-2505

