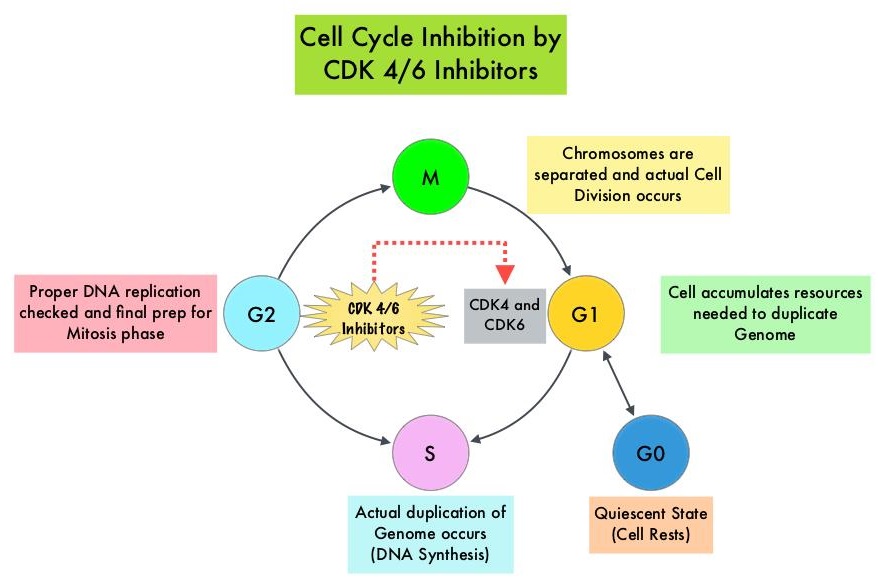
Cyclin Dependent Kinases 4 and 6 (CDK4 and CDK6) phosphorylate RetinoBlastoma protein (RB), and initiate transition from the G1 phase to the S phase of the cell cycle. RetinoBlastoma protein has antiproliferative and tumor-suppressor activity and phosphorylation of RB protein nullifies its beneficial activities. CDK4 and CDK6 are activated in hormone receptor positive breast cancer, promoting breast cancer cell proliferation. Further, there is evidence to suggest that endocrine resistant breast cancer cell lines depend on CDK4 for cell proliferation. The understanding of the role of Cyclin Dependent Kinases in the cell cycle, has paved the way for the development of CDK inhibitors.
Even though major international oncology treatment guidelines recommend a sequence of endocrine based therapies with or without targeted therapies in postmenopausal women with HR-positive, HER2-negative metastatic breast cancer, Real-World Data suggests that upfront use of chemotherapy remains common even in the absence of visceral crisis. This treatment approach may partly be due to paucity of data directly comparing hormonal therapies with chemotherapy regimens, in this patient group. To provide guidance with additional evidence, the authors conducted a comprehensive systematic review and network meta-analysis to evaluate the efficacy and activity of several first or second line hormonal therapy and chemotherapy regimens that have been investigated in randomized controlled trials, and the researchers aimed to compare these two different approaches.
This analysis included all Phase II and III randomized controlled trials investigating chemotherapy with or without targeted therapies and hormone therapies with or without targeted therapies as first-line or second-line treatments, or both, in postmenopausal women with HR-positive, HER2-negative metastatic breast cancer. Relevant examples of new targeted therapies are mTOR inhibitor Everolimus (AFINITOR®), CDK4/6 inhibitors Palbociclib (IBRANCE®), Ribociclib (KISQALI®) and Abemaciclib (VERZENIO®), and PI3K inhibitor Alpelisib (PIQRAY®), which are used in combination with endocrine therapy. Following a literature search on PubMed, Embase, Cochrane Central Register of Clinical Trials, Web of Science, and online archives of the most relevant international oncology conferences published between Jan 1, 2000 and Dec 31, 2017, 140 studies were selected, comprising of 50,029 patients. Studies exclusively enrolling premenopausal patients and those with HER2-positive or triple-negative breast cancer were excluded from this analysis. The median age was 58 yrs and median follow up was 20 months. All treatments were compared to Anastrozole (ARIMIDEX®) and to CDK4/6 inhibitor Palbociclib (IBRANCE®) plus Letrozole (FEMARA®). The Primary outcome was Progression Free Survival (PFS) and the Secondary outcome was Overall Response Rate.
In this analysis, it was noted that CDK4/6 inhibitors and PIK3K inhibitor (in patients with PIK3CA mutation) along with endocrine therapy was superior to standard endocrine therapy such as Anastrozole alone or Fulvestrant (FASLODEX®) alone, with significantly better PFS. Chemotherapy regimens with or without targeted agents were not significantly better than CDK4/6 inhibitors plus endocrine therapy. Further, the combination of CDK4/6 inhibitors plus endocrine therapy was associated with a favorable toxicity profile compared to chemotherapy. There were no significant differences noted in PFS among the three CDK4/6 inhibitors in combination with an Aromatase Inhibitor or Fulvestrant.
The authors concluded that in the first and second line setting, CDK4/6 inhibitors plus endocrine therapies are superior to standard single agent endocrine therapies in terms of Progression Free Survival. Moreover, no chemotherapy regimen with or without targeted therapy is significantly better than CDK4/6 inhibitors plus endocrine therapies in terms of Progression Free Survival. The researchers added that this is the first study to directly compare all three CDK4/6 inhibitors combined with an Aromatase Inhibitor or Fulvestrant. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Giuliano M, Schettini F, Rognoni C, et al. Lancet Oncol. 2019;20:1360-1369.

